Is Remdesivir Drug Fda-approved For Covid-19 Treatment
This drug is. GILD today announced that the US.
 Fda Approves Remdesivir For Covid 19 Treatment
Fda Approves Remdesivir For Covid 19 Treatment
The FDA approved remdesivir to be used on hospitalized COVID-19 patients age 12 and older and weighing at least 88 pounds.
Is remdesivir drug fda-approved for covid-19 treatment. 22 the Food and Drug Administration FDA approved the first drug for treating COVID-19. One drug that is. Veklury remdesivir was approved by the FDA on October 22 2020.
Veklury remdesivir is a SARS-CoV-2 nucleotide analog RNA polymerase inhibitor indicated for the treatment of COVID-19 patients who require hospitalization. On Thursday October 22 the Food and Drug Administration approved remdesivir to treat patients with COVID-19 who have been hospitalized. This makes remdesivir the first fully approved.
Patients 12 years of age and older can be. 1 day agoThe drug is a patented antiviral drug manufactured by Gilead US initially developed for the treatment of viral hepatitis and a cold-like virus called respiratory syncytial virus RSV. According to AIIMS director Randeep Guleria the timing of using these drugs.
CNN The US Food and Drug Administration has approved remdesivir for the treatment of coronavirus infection the drugs maker Gilead Sciences. Today the Food and Drug Administration FDA approved remdesivir as the first COVID-19 treatment for use in hospitals and other health care facilities. Early effective treatment of any disease can help avert progression to more serious illness especially for patients at high risk of disease progression and severe illness with the additional benefit of reducing the burden on healthcare systems.
FabiFlu is a drug that is approved by DGCI for the treatment of COVID-19. FOSTER CITY Calif--BUSINESS WIRE--Oct. FDA Approves Veklury remdesivir for the Treatment of COVID-19.
22 2020-- Gilead Sciences Inc. With reports of extreme shortages of ventilator beds medical oxygen Remdesivir Tocilizumab and other important medicines required emergency treatment the country is experiencing utter chaos and despair. Today the US.
Remdesivir an antiviral medication given intravenously is now. The recommended dose of this medication is 1800 mg dose twice on day 1 and 800 mg dose twice till day 14. Food and Drug Administration issued an emergency use authorization for the investigational antiviral drug remdesivir for the treatment of.
Food and Drug Administration FDA has approved the antiviral drug Veklury remdesivir for the treatment of patients with COVID-19 requiring hospitalization. The Food and Drug Administration on Thursday approved the antiviral drug remdesivir as a treatment for patients with COVID-19. Remdesivir is an FDA-approved and sold under the brand name Veklury intravenous antiviral drug for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kilogram.
Today FDA approved Veklury remdesivir the first drug approved to treat COVID-19 for use in adults and pediatric patients 12 years of age and. Remdesivir steroids plasma Tocilizumab and Favipiravir are some of the most widely used treatments for COVID-19. Exit Full Screen.
FDA has approved one drug remdesivir Veklury for the treatment of COVID-19 in certain situations. The second wave of COVID-19 has resulted in an unprecedented rise in cases. Food and Drug Administration approved the antiviral drug Veklury remdesivir for use in adult and pediatric patients 12 years of age and older.
Due to its initial response against the virus the US FDA approved Remdesivir to treat COVID-19. This is a more specific age demographic than those permitted to receive.
 Dod Funded Drug Remdesivir Receives Fda Approval For Covid 19 Treatment
Dod Funded Drug Remdesivir Receives Fda Approval For Covid 19 Treatment
 Gilead Suspends Access To Experimental Covid 19 Drug Remdesivir Stat
Gilead Suspends Access To Experimental Covid 19 Drug Remdesivir Stat
 Remdesivir Drug Hailed As Treatment For Covid 19 Following Fda Approval Abc7 Chicago
Remdesivir Drug Hailed As Treatment For Covid 19 Following Fda Approval Abc7 Chicago
 Remdesivir Price Still A Puzzle To Be Solved By Gilead Sciences Shots Health News Npr
Remdesivir Price Still A Puzzle To Be Solved By Gilead Sciences Shots Health News Npr
 What Is Remdesivir How Is It Used For Covid 19 Covid 19 Featured Health Topics Hackensack Meridian Health
What Is Remdesivir How Is It Used For Covid 19 Covid 19 Featured Health Topics Hackensack Meridian Health
 Fda Approves Remdesivir As First Drug To Treat Covid 19 Coronavirus Outbreak News The Indian Express
Fda Approves Remdesivir As First Drug To Treat Covid 19 Coronavirus Outbreak News The Indian Express
 Remdesivir S Fda Approval To Treat Covid 19 Sets It Ahead Of Treatment Pack
Remdesivir S Fda Approval To Treat Covid 19 Sets It Ahead Of Treatment Pack
 Q A How To Request Remdesivir For Patients With Covid 19
Q A How To Request Remdesivir For Patients With Covid 19
 Remdesivir Approved By The Fda To Treat Covid 19 News Cardiff University
Remdesivir Approved By The Fda To Treat Covid 19 News Cardiff University
 Fda Approves Remdesivir For Treatment Of Covid 19
Fda Approves Remdesivir For Treatment Of Covid 19
 Remdesivir First Therapeutic Approved By Fda For Covid 19 Rt
Remdesivir First Therapeutic Approved By Fda For Covid 19 Rt
Fda Approves First Drug As Treatment For Covid 19
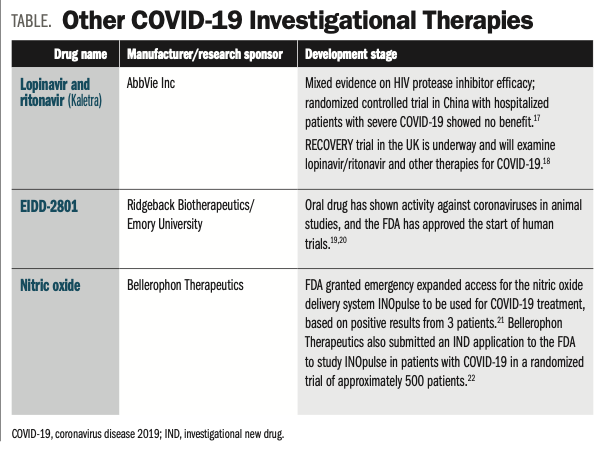 Investigational Drugs In The Pipeline For Covid 19
Investigational Drugs In The Pipeline For Covid 19
 What Remdesivir Approval By Fda Means
What Remdesivir Approval By Fda Means
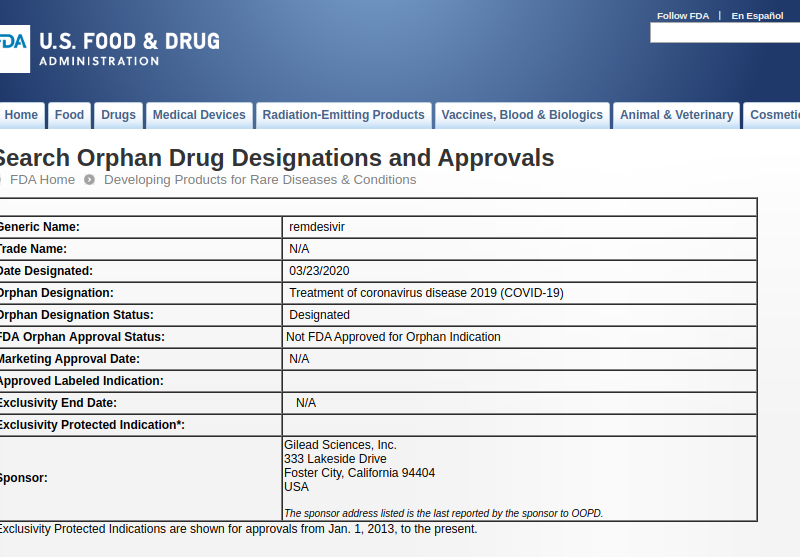 Fda Gives Gilead A Seven Year Regulatory Monopoly For Remdesivir To Treat Covid 19 On Grounds It Is An Orphan Treating A Rare Disease Knowledge Ecology International
Fda Gives Gilead A Seven Year Regulatory Monopoly For Remdesivir To Treat Covid 19 On Grounds It Is An Orphan Treating A Rare Disease Knowledge Ecology International
 Doctors Lambaste Process For Distributing Covid 19 Drug Remdesivir Stat
Doctors Lambaste Process For Distributing Covid 19 Drug Remdesivir Stat
Fda Approve Antiviral Remdesivir Drug For Covid 19 Treatment
Post a Comment for "Is Remdesivir Drug Fda-approved For Covid-19 Treatment"