Which Covid-19 Vaccines Are In Phase 3 Trials
MRNA Phase 3 status. Pfizer and BioNTech Announce Publication of Results from Landmark Phase 3 Trial of BNT162b2 COVID-19 Vaccine Candidate in The New England Journal of Medicine.
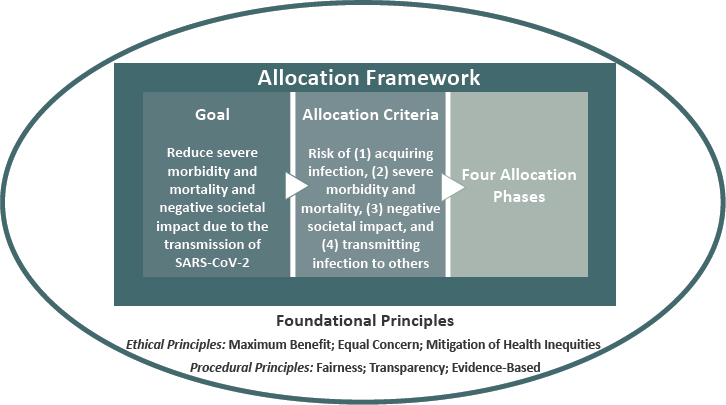 3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press
Type of vaccine.
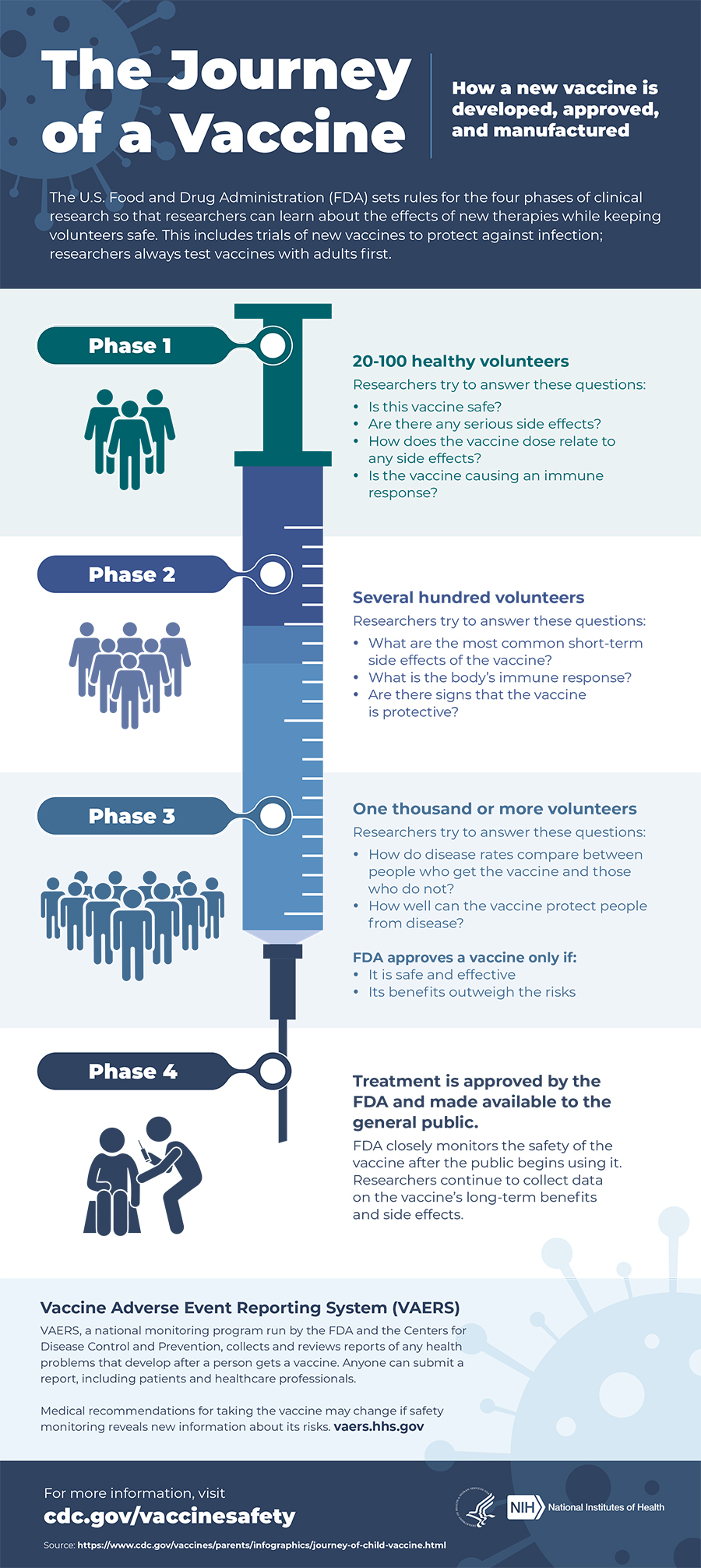
Which covid-19 vaccines are in phase 3 trials. 2 days agoValneva Initiates Pivotal Phase 3 Clinical Trial for its Inactivated COVID-19 Vaccine Candidate using Dynavaxs CpG 1018 Adjuvant - Phase 3 clinical trial to enroll approximately 4000. PFE and BioNTech SE Nasdaq. Federal health researchers and vaccine maker Novavax announced Monday that they will begin a Phase 3 trial for Novavaxs COVID-19 vaccine candidate in.
Moderna has recently announced a COVID-19 vaccine candidate mRNA-1273. Thursday December 10 2020 - 1021am. A vaccine candidate for COVID-19 from Chinas Sinovac Biotech on display at the China International Fair for Trade in Services in Beijing on September 6.
2 days agoThe interim results of the Phase-3 trials for Covaxin have shown a clinical efficacy of 78 against mild moderate and severe Covid-19 disease makers Bharat Biotech and Indian Council of. Data from 43448 participants half of whom received BNT162b2 and half of whom received placebo showed that the vaccine candidate was well tolerated and demonstrated 95 efficacy in. In July Medicago launched Phase 1 trials on a plant-based Covid-19 vaccine in combination with adjuvants to boost the immune systems response to the viral proteins.
This vaccine relies on RNA to kickstart the endogenous production of proteins similar enough to the virus that they trigger the bodys adaptive immune system to produce antibodies. 1 Clinical trial identifier. Vaccines in Phase 3 Clinical Trials As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States.
Analysis of the data indicates a vaccine efficacy rate of 95 p. Ongoingrecruiting Number of doses. Limited BE today announced that it has successfully completed the phase III clinical trials of its COVID-19 subunit vaccine candidate in IndiaThe company has also received approval to start phase III clinical trials from the Central Drugs Standard Control Organization CDSCO Subject Expert Committee.
3 hours agoThe Phase III clinical study to be conducted in 15 sites across India will evaluate the Immunogenicity and Safety of BEs SARS-CoV-2 COVID-19 vaccine for protection against COVID-19. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine. Phase 3 trial of Novavax investigational COVID-19 vaccine opens NIH- and BARDA-funded trial will enroll up to 30000 volunteers.
The Phase 3 results of the clinical trials of COVAXIN Indias first indigenous COVID-19 vaccine showed an overall interim clinical efficacy of 78 and 100 efficacy against severe COVID-19. By Erika Edwards A fourth Covid-19 vaccine candidate has gone into the final stage of clinical trials in the US with Johnson Johnson announcing the. Hyderabad-based vaccine and pharmaceutical company Biological E.
French vaccine maker Valneva to launch Phase 3 trial of COVID-19 vaccine its making with Dynavax after positive results Valneva said on. Saint-Herblain France April 21 2021 Valneva SE a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need today announced it has initiated a pivotal Phase 3 clinical trial for its inactivated adjuvanted COVID-19 vaccine candidate VLA2001. NEW YORK MAINZ Germany--BUSINESS WIRE-- Pfizer Inc.
A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun. The vaccine known as mRNA-1273 was co-developed by the Cambridge Massachusetts-based biotechnology company Moderna Inc and the National Institute of Allergy and Infectious Diseases NIAID part of the National Institutes of. BNTX today announced that after conducting the final efficacy analysis in their ongoing Phase 3 study their mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints.
The Department of Defense is withdrawing funding for a phase 3 trial of Inovios COVID-19 vaccine candidate INO-4800 due to rapid deployment of other vaccines.
 Safety And Efficacy Of An Rad26 And Rad5 Vector Based Heterologous Prime Boost Covid 19 Vaccine An Interim Analysis Of A Randomised Controlled Phase 3 Trial In Russia The Lancet
Safety And Efficacy Of An Rad26 And Rad5 Vector Based Heterologous Prime Boost Covid 19 Vaccine An Interim Analysis Of A Randomised Controlled Phase 3 Trial In Russia The Lancet
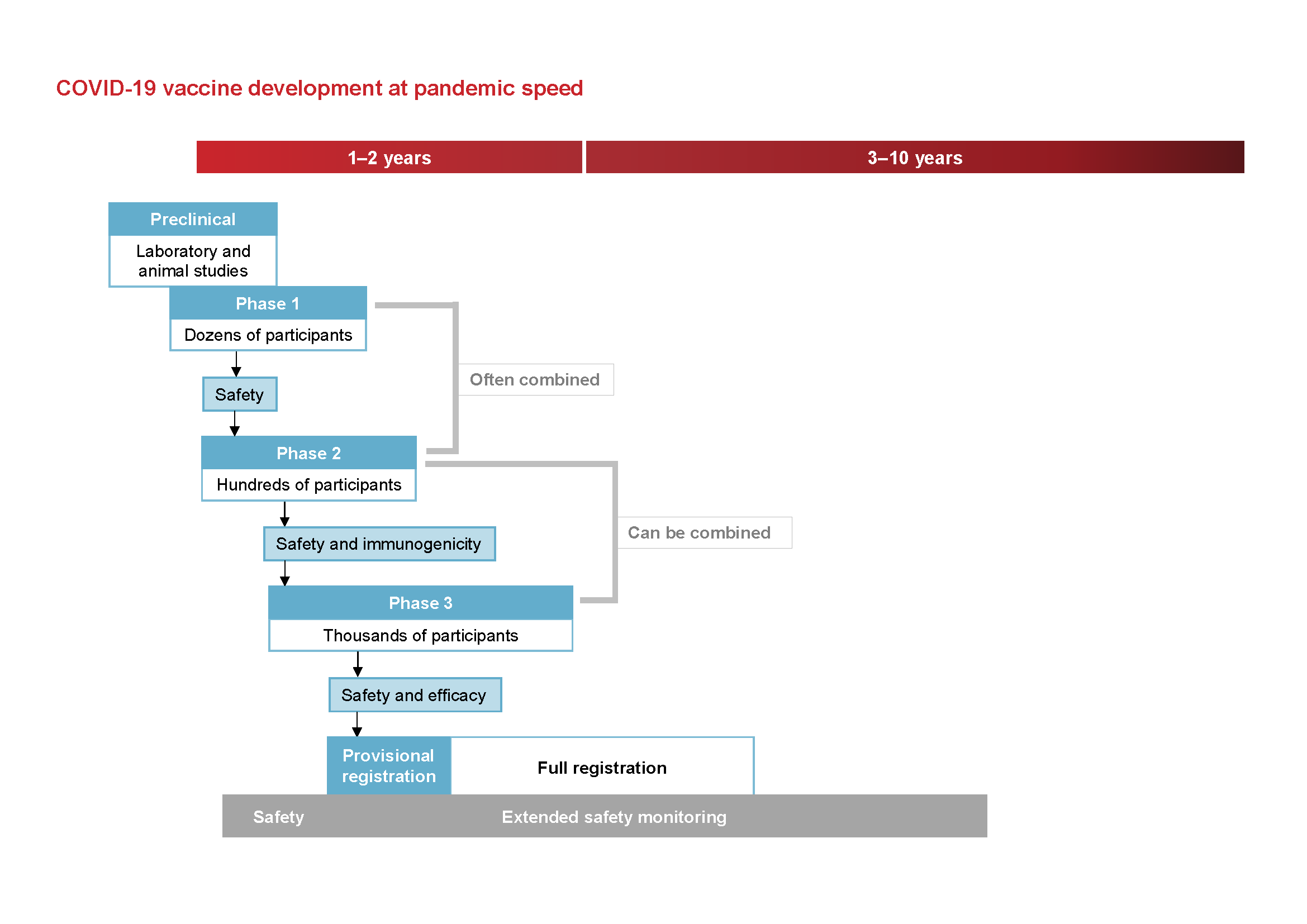 Phases Of Clinical Trials Ncirs
Phases Of Clinical Trials Ncirs
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Vaccines Nih Covid 19 Research
Vaccines Nih Covid 19 Research
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
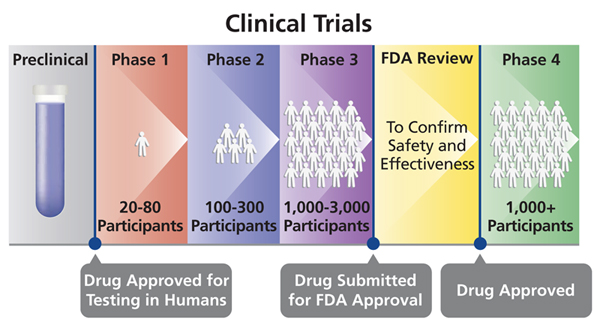 First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
Welcome To The Official Website Of Iowa County Wivaccine Information
 Delays In Clinical Trials Present Opportunities For Pharma Companies To Evolve Cortellis
Delays In Clinical Trials Present Opportunities For Pharma Companies To Evolve Cortellis
 The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
 Nasem S Draft Priorities For Covid 19 Vaccine News About Energy Storage Batteries Climate Change And The Environment
Nasem S Draft Priorities For Covid 19 Vaccine News About Energy Storage Batteries Climate Change And The Environment
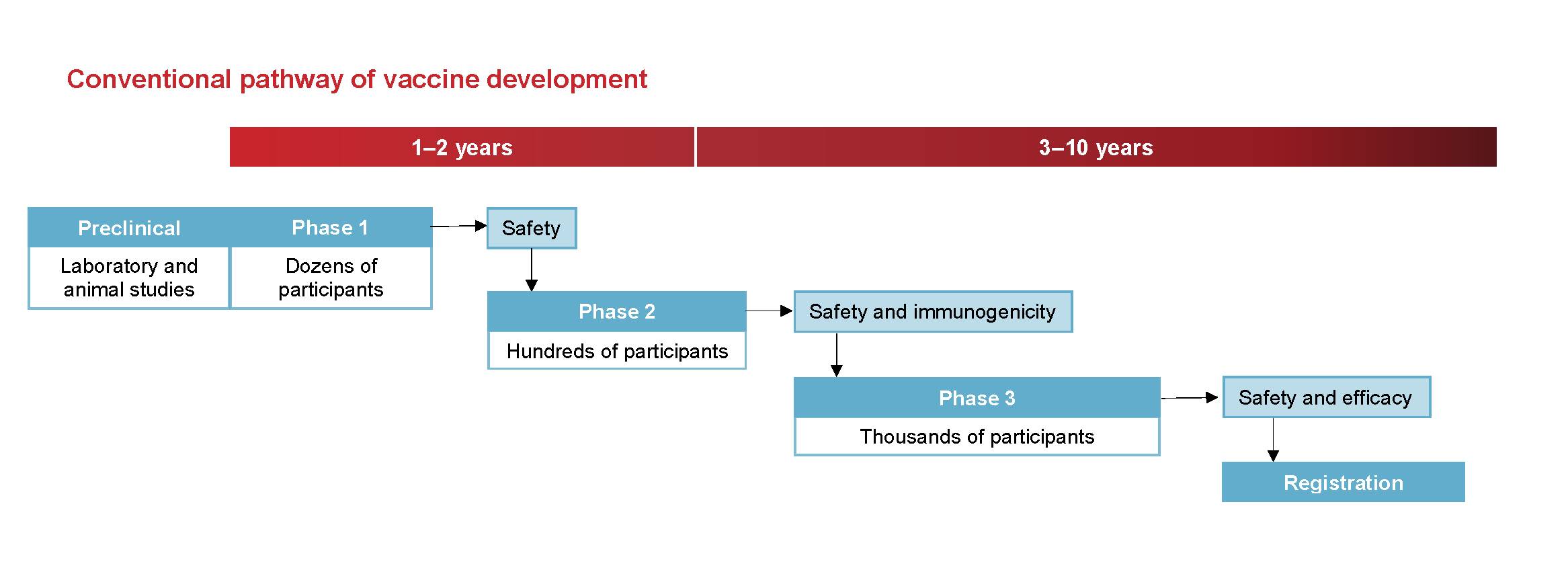 Phases Of Clinical Trials Ncirs
Phases Of Clinical Trials Ncirs
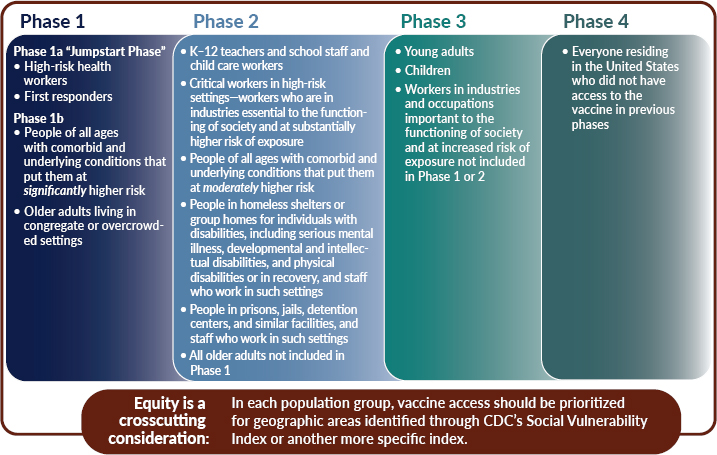 3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press
3 A Framework For Equitable Allocation Of Covid 19 Vaccine Framework For Equitable Allocation Of Covid 19 Vaccine The National Academies Press
 The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021

Post a Comment for "Which Covid-19 Vaccines Are In Phase 3 Trials"