Coronavirus Vaccine Phase 3 Data
18 hours agoIn clinical trials both the two-dose COVID-19 vaccines Covishield and Covaxin being used have been shown to lower the risk of death severe disease and mild infections in vaccinated individuals even after only the first dose. After those studies yielded promising results Phase 3 testing.
 How Close Is A Coronavirus Vaccine Free To Read Financial Times
How Close Is A Coronavirus Vaccine Free To Read Financial Times
Both Moderna and Pfizer COVID-19 vaccines have immunity that lasts for at.
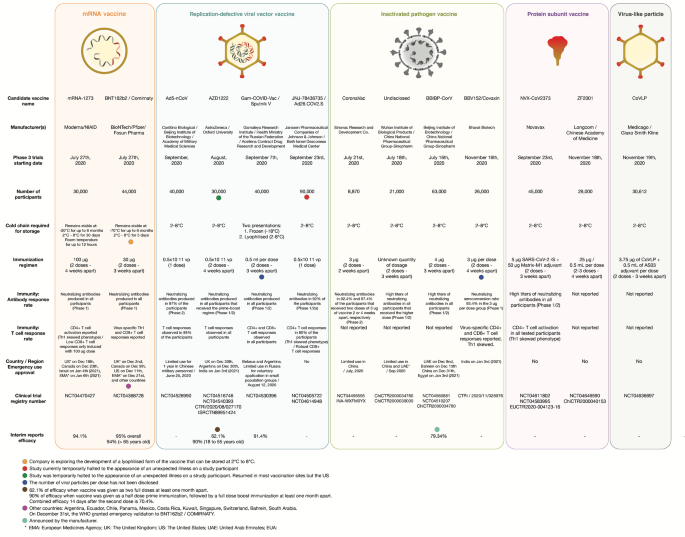
Coronavirus vaccine phase 3 data. Some of this information comes from phase three clinical trial data. Thats how phase 3 trial is designed. The Phase 3 trial is designed as a 11 vaccine candidate to placebo randomized observer-blinded study to obtain safety immune response and efficacy data needed for regulatory review.
170 confirmed cases of COVID-19 were evaluated with 162 observed in the placebo group versus 8 in the vaccine. 2 days agoThe primary endpoint of the Phase 3 clinical trial is based on the first occurrence of PCR-confirmed symptomatic mild moderate or severe COVID-19. NEW BRUNSWICK NJ April 21 2021 Johnson Johnson the Company today announced publication in the New England Journal of Medicine of primary data from the Phase 3 ENSEMBLE clinical trial for its single-dose COVID-19 vaccine developed by the Janssen Pharmaceutical Companies of Johnson Johnson Janssen.
Vertex Amend its Collaboration with CRISPR Therapeutics for Development and Commercialization of CTX001 in SCD and Beta Thalassemia. Zydus Cadila says its ZyCoV-D vaccine had very good results in Phase 1 and 2 trials. An investigational COVID-19 vaccine developed by Janssen Pharmaceuticals appears to be safe and effective at preventing moderate and severe COVID-19 in adults according to an interim analysis of Phase 3 clinical data conducted Jan.
This study is consistent with the occurrence of adverse reactions observed in the phase III clinical studies outside China but compared with the interim analysis results of the overseas phase III clinical trials the incidence of adverse reactions in this study is significantly lower than the interim analysis results of. Summary of Data from Phase 3 Clinical Trial The primary efficacy analysis population referred to as the Per-Protocol Set included 28207 participants who received two doses at 0 and 1 month of either Moderna COVID19 Vaccine n14134 or placebo n14073 and had a negative baseline SARSCoV2 status. Interim Analysis of Phase 3 Clinical Data Released.
Safety data on large-scale emergency use of SINOPHARM COVID-19 Vaccine. Dosing of participants in Phase 3 has been completed and the data analysis process is. Claritas Collaborates with CMAX to Initiate P-I Study of R-107 for Pulmonary Arterial Hypertension.
Published Phase III trial results for Covishield showed a 70 vaccine efficacy in preventing symptomatic COVID-19 and 100 efficacy in preventing severe. Janssen Investigational COVID-19 Vaccine. Pharma Biotech Biosimilars COVID-19.
The publication of the primary analysis follows the topline efficacy and. One of the latest Valneva pushed VLA2001 into a pivotal UK study yesterday while Ocugen hopes that its contender Covaxin could get the nod in the US on the back of data from a phase 3 trial in. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate Meeting All Primary Efficacy Endpoints Wednesday November 18 2020 - 0659am Primary efficacy analysis demonstrates BNT162b2 to be 95 effective against COVID-19 beginning 28 days after the first dose.
As the world awaits pivotal US data on Novavaxs Covid-19 jab plenty of other players are still trying to get in on the second wave of vaccines. Last March the scientists were the first to put a Covid-19 vaccine into human trials.
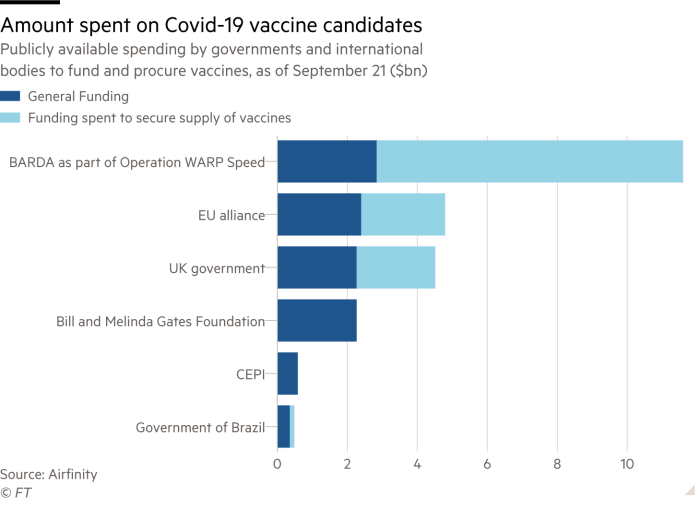 How Close Is A Coronavirus Vaccine Free To Read Financial Times
How Close Is A Coronavirus Vaccine Free To Read Financial Times
 Coronavirus Information Eastham Ma
Coronavirus Information Eastham Ma
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
 Latest Cdc Panel Recommends Resuming Use Of Johnson Johnson Vaccine
Latest Cdc Panel Recommends Resuming Use Of Johnson Johnson Vaccine
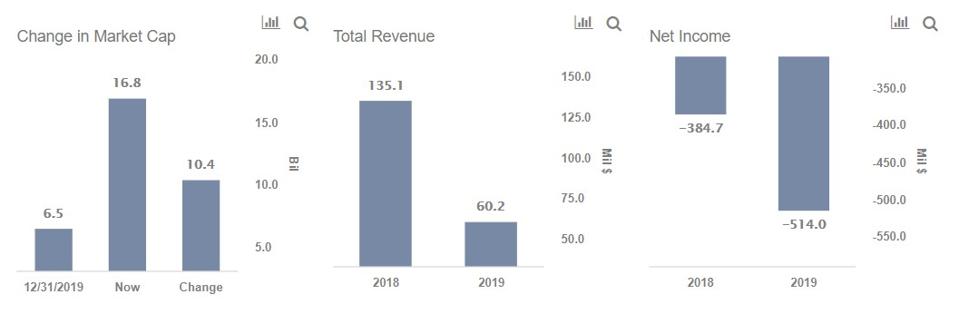 Best Coronavirus Vaccine Stock Moderna Inovio Sanofi Or Johnson Johnson
Best Coronavirus Vaccine Stock Moderna Inovio Sanofi Or Johnson Johnson
 Coronavirus Vaccine Bharat Biotech Recruits 13 000 Volunteers In Phase 3 Trials
Coronavirus Vaccine Bharat Biotech Recruits 13 000 Volunteers In Phase 3 Trials
Welcome To The Official Website Of Iowa County Wivaccine Information
Sinopharm S Covid 19 Vaccine Scores Approval In China Pmlive
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Https Www Alabamapublichealth Gov Covid19 Assets Cov Pfizer Moderna Vaccine Trial Summary Pdf
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Russia Coronavirus Vaccine 91 6 Effective Lancet
 Pfizer Covid Vaccine Is 95 Effective Plans To Submit To Fda In Days
Pfizer Covid Vaccine Is 95 Effective Plans To Submit To Fda In Days
 The Clinical Trial Results Stampede Begins Covid 19 Vaccine Race Month 7 Absolutely Maybe
The Clinical Trial Results Stampede Begins Covid 19 Vaccine Race Month 7 Absolutely Maybe
 Coronavirus Vaccine How Pfizer Covid 19 Vaccine Was Developed In Record Time
Coronavirus Vaccine How Pfizer Covid 19 Vaccine Was Developed In Record Time
 Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021

Post a Comment for "Coronavirus Vaccine Phase 3 Data"