Coronavirus Vaccine Trials Phase 3
Novavax on Monday announced the start of the Phase 3 trial of its Covid-19 vaccine in the United States and Mexico. French vaccine maker Valneva to launch Phase 3 trial of COVID-19 vaccine its making with Dynavax after positive results Valneva said on.
 Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
Covid 19 Vaccine Race Month 6 First Emergency Use Phase 3 Trials Absolutely Maybe
Interim Analysis of Phase 3 Clinical Data Released.

Coronavirus vaccine trials phase 3. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. Ocugen NASDAQOCGN has unveiled the results of a phase 3. COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov.
Data from Phase 3 clinical trial confirm vaccine is effective. Saint-Herblain France April 21 2021 Valneva SE a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need today announced it has initiated a pivotal Phase 3 clinical trial for its inactivated adjuvanted COVID-19 vaccine candidate VLA2001. CNN British drugmaker AstraZeneca said Monday it has started Phase 3 trials of its experimental coronavirus vaccine in the United States becoming the.
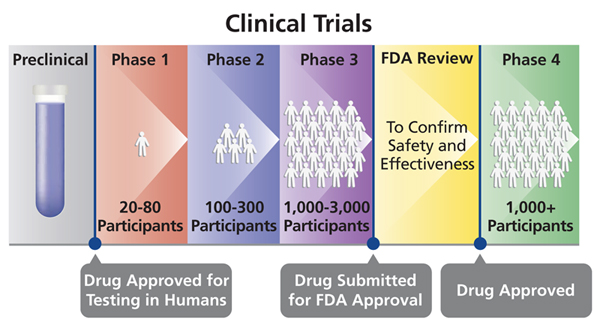
Last March the scientists were the first to put a Covid-19 vaccine into human trials. Phase 3 clinical trial of COVID-19 vaccine underway August 6 2020 As the race to develop a safe and effective vaccine to protect against COVID-19 continues phase 3 trials of investigational vaccines are underway. Vaccine trials usually undergo three rounds of testing.
Trump says every American can get a coronavirus vaccine by. The Phase 3 trial is being conducted in collaboration with Operation Warp Speed the federal governments coronavirus vaccine effort. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine Learn more about US.
The vaccine known as mRNA-1273 was co-developed by the Cambridge Massachusetts-based biotechnology company Moderna Inc and the National Institute of Allergy and Infectious Diseases NIAID part of the. Health advocates go door-to-door to fight vaccine hesitancy Savannah Georgia CNN The first Phase 3 clinical trial of a coronavirus vaccine in. 1 day agoA new coronavirus vaccine candidate could soon be authorized for use if its excellent clinical results are any indication.
After those studies yielded promising results Phase 3 testing. The interim results of the Phase-3 trials for Covaxin have shown a clinical efficacy of 78 against mild moderate and severe. An investigational COVID-19 vaccine developed by Janssen Pharmaceuticals appears to be safe and effective at preventing moderate and severe COVID-19 in adults according to an interim analysis of Phase 3 clinical data conducted Jan.
It is the fifth company to launch a large-scale trial of a coronavirus vaccine. A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun. 1 day agoThe Phase 3 trial Cov-Compare VLA2001-301 will compare Valnevas SARS-CoV-2 vaccine candidate VLA2001 against AstraZenecas conditionally approved vaccine Vaxzevria in a.
The first two trials are typically smaller ones testing mostly for vaccine safety and biological activity. Phase 1 phase 2 and phase 3. What The investigational vaccine known as mRNA-1273 was 941 efficacious in preventing symptomatic coronavirus disease 2019 COVID-19 according to preliminary results from a Phase 3 clinical trial reported in the New England Journal of Medicine.
French biotech company Valneva has launched a pivotal Phase 3 trial for its experimental COVID-19 vaccine the last step before seeking regulatory approval. It comes as Valneva said it would. 1 day agoThe interim analysis was based on 87 symptomatic cases of Covid-19.
Janssen Investigational COVID-19 Vaccine.
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 China Begins Phase I Clinical Trial Of Covid 19 Vaccine
China Begins Phase I Clinical Trial Of Covid 19 Vaccine
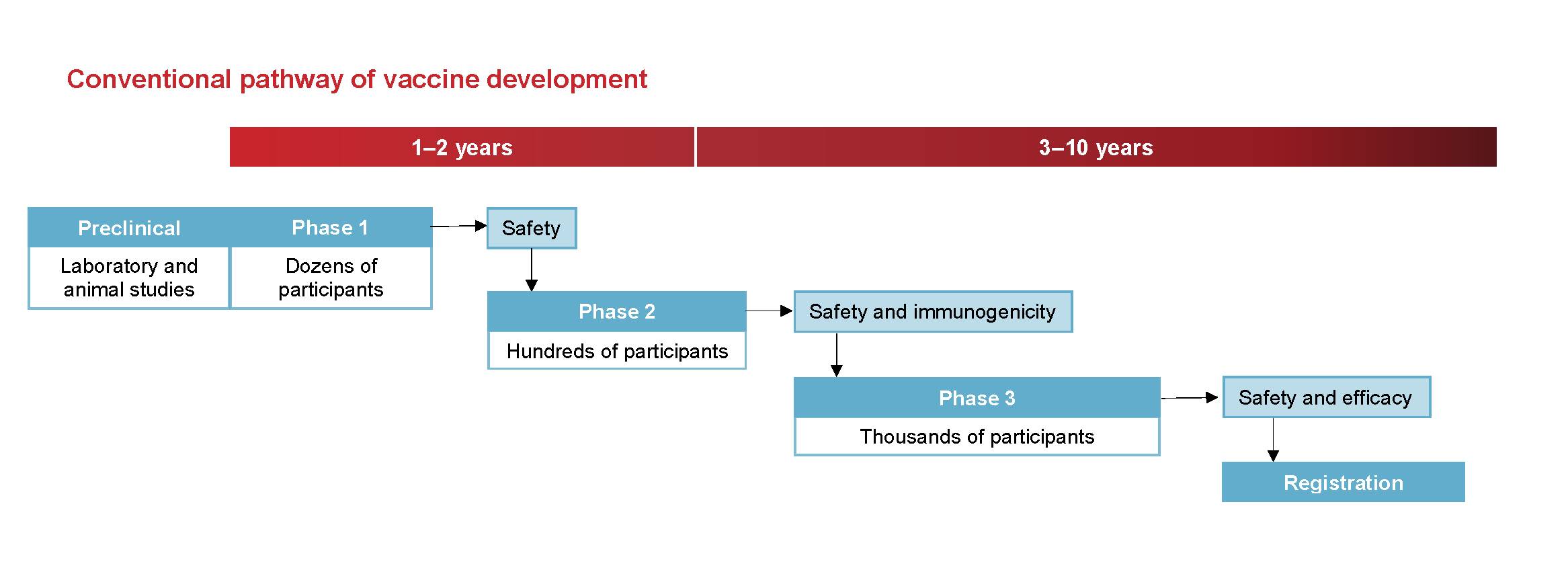 Phases Of Clinical Trials Ncirs
Phases Of Clinical Trials Ncirs
 Covid 19 Three In Five Worry About Side Effects Of A Vaccine Many Plan To Take A Wait And See Approach Angus Reid Institute
Covid 19 Three In Five Worry About Side Effects Of A Vaccine Many Plan To Take A Wait And See Approach Angus Reid Institute
 Novavax Phase 3 Covid 19 Vaccine Trial Completes Enrollment In Two Months
Novavax Phase 3 Covid 19 Vaccine Trial Completes Enrollment In Two Months
 3 000 Volunteers In Phase 3 Clinical Trial For Covid 19 Vaccine Pm Muhyiddin
3 000 Volunteers In Phase 3 Clinical Trial For Covid 19 Vaccine Pm Muhyiddin
 Uk Leads Rapid Delivery Of Novavax Phase 3 Covid 19 Vaccine Trial
Uk Leads Rapid Delivery Of Novavax Phase 3 Covid 19 Vaccine Trial
 The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
 Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health

 First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
First In Human Covid 19 Vaccines Tales Of Phase 1 Clinical Trials Past Absolutely Maybe
 Coronavirus Vaccine 90 Effective Say Pfizer And German Company Biontech News Dw 09 11 2020
Coronavirus Vaccine 90 Effective Say Pfizer And German Company Biontech News Dw 09 11 2020
 World S First Participants For Novavax Phase 3 Covid 19 Vaccine Trial Enroll Through Nihr Patient Recruitment Centre
World S First Participants For Novavax Phase 3 Covid 19 Vaccine Trial Enroll Through Nihr Patient Recruitment Centre
 S Korea Laggard In The Race To Bring Covid 19 Vaccine To Market Pulse By Maeil Business News Korea
S Korea Laggard In The Race To Bring Covid 19 Vaccine To Market Pulse By Maeil Business News Korea
 Coronavirus Vaccine Covishield Completes Enrolment Of Phase 3 Clinical Trials Under Icmr Serum Institute S Partnership Business Insider India
Coronavirus Vaccine Covishield Completes Enrolment Of Phase 3 Clinical Trials Under Icmr Serum Institute S Partnership Business Insider India
 Russia Approves Phase Iii Trial Of Cansino Covid 19 Vaccine
Russia Approves Phase Iii Trial Of Cansino Covid 19 Vaccine
 Covid 19 Vaccines November Update Progress Of Clinical Trials Post
Covid 19 Vaccines November Update Progress Of Clinical Trials Post
 About Our Ensemble Studies Johnson Johnson
About Our Ensemble Studies Johnson Johnson
 Coronavirus Explained What Are The Phases In The Clinical Trials Of The Covid 19 Vaccine Caoronavirus Vaccine
Coronavirus Explained What Are The Phases In The Clinical Trials Of The Covid 19 Vaccine Caoronavirus Vaccine
Post a Comment for "Coronavirus Vaccine Trials Phase 3"