Covid Monoclonal Antibody Clinical Trials
To volunteer sign up for the CoVPN Volunteer Screening Registry. UB launches monoclonal antibody study for household members of anyone who recently tested positive Submitted Tue Nov 10th 2020 0425 pm.
 Covid 19 Archives The Antibody Society
Covid 19 Archives The Antibody Society
1 day agoThe trial is a randomized double-blind adaptive study that will examine the clinical safety and efficacy of SAB-185 in addition to standard of care in non-hospitalized patients with mild to moderate COVID-19 at risk for disease progression.

Covid monoclonal antibody clinical trials. Professor of Healthcare Policy and Research Dr. Two Phase 3 randomized placebo-controlled double-blind clinical trials testing whether experimental monoclonal antibodies mAbs can prevent infection by SARS-CoV-2 coronavirus are now enrolling. Several neutralizing monoclonal antibodies mAbs to severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 have been developed and are now under evaluation in clinical trials.
Glesby who is also director of the Cornell HIV Clinical Trials Unit is leading a clinical trial testing a drug designed to prevent the complications from COVID-19 that result when the bodys immune system goes into overdrive. Clinical trials offer hope for many people and provide an opportunity to help researchers find new or improve existing treatments. With green light from the FDA Rockefeller scientists started human trials this week for a new monoclonal antibody drug as a potential treatment for COVID-19.
If you are 18 years of age or older and in good health you may be able to participate. If you have had COVID-19 in the past you can donate plasma or blood to help others recover. The Inpatient Treatment with Anti-Coronavirus Immunoglobulin ITAC clinical trial is evaluating the investigational antiviral Veklury remdesivir developed by Gilead Sciences Inc in combination with hyperimmune intravenous immunoglobulin hIVIG for adult patients hospitalized for medical management of COVID-19 without related serious end-organ failure.
COVID-19 Study Assessing the Efficacy and Safety of Anti-Spike SARS CoV-2 Monoclonal Antibodies for Prevention of SARS CoV-2 Infection Asymptomatic in Healthy Adults and Adolescents Who Are Household Contacts to an Individual With a Positive SARS-CoV-2 RT-PCR Assay - Full Text View - ClinicalTrialsgov. The COVID-19 Prevention Network CoVPN is conducting studies to find safe and effective vaccines and monoclonal antibodies for SARS-CoV-2 the virus that causes COVID-19. The two experimental antibodies BRII-196 and BRII-198 target SARS-CoV-2 the virus that causes COVID-19.
COVID-19 prevention clinical research studies evaluating vaccines and monoclonal antibodies in adults are now enrolling. JOIN A TREATMENT CLINICAL TRIAL. The National Institutes of Health announced the start of two clinical trials for a potential coronavirus treatment called monoclonal antibodies.
A clinical trial testing the safety and efficacy of an investigational monoclonal antibody for treating people who are hospitalized with respiratory disease and low blood oxygen due to infection with SARS-CoV-2 the virus that causes COVID-19 has begun. COVID-19 Clinical Trials. Learn more about COVID-19 therapeutic options and ongoing clinical trials.
Clinical trials use volunteers who agree to participate in these types of studies. Clinical trials are medical research studies to test new ways to prevent detect or treat diseases. Clinical trials of monoclonal antibodies to prevent COVID-19 now enrolling.
Rockefeller begins testing of new COVID-19 antibody drug in people January 14 2021 Monoclonal antibodies attaching to coronaviruses. 22 hours agoCOVID-19 clinical trial. A Phase 23 clinical trial has begun to evaluate a combination investigational monoclonal antibody therapy for its safety and efficacy in people who have mild or moderate COVID-19.
Has participated or is participating in a clinical research study evaluating COVID-19 convalescent plasma mAbs against SARS-CoV-2 eg bamlanivimab or intravenous immunoglobulin IVIG within 3 months or within 5 half-lives of the investigational product whichever is longer prior to the screening visit. Each sub-study in ACTIV-2 shares the placebo group and plans to enroll 110 participants. Image of an antibody binding to the surface of a virus blocking entry into a human cell.
The trial known as ACTIV-2 is sponsored by the National Institute of Allergy and Infectious Diseases. This is a first-in-human phase one study to test the safety and tolerability of LY3819253 LY-CoV555 when it is given by injection into a vein to participants hospitalized with COVID-19. If youve never had COVID-19 you can join a clinical trial for these vaccines or for other ways to prevent the disease.
Two trials being conducted through CoVPN will test monoclonal antibodies for their ability to prevent COVID-19 in uninfected adults who are at high risk of acquiring SARS-CoV-2 due to living or working with infected individuals. Blood tests will be done to check how much LY3819253 is in the bloodstream and how long the body takes to eliminate it.
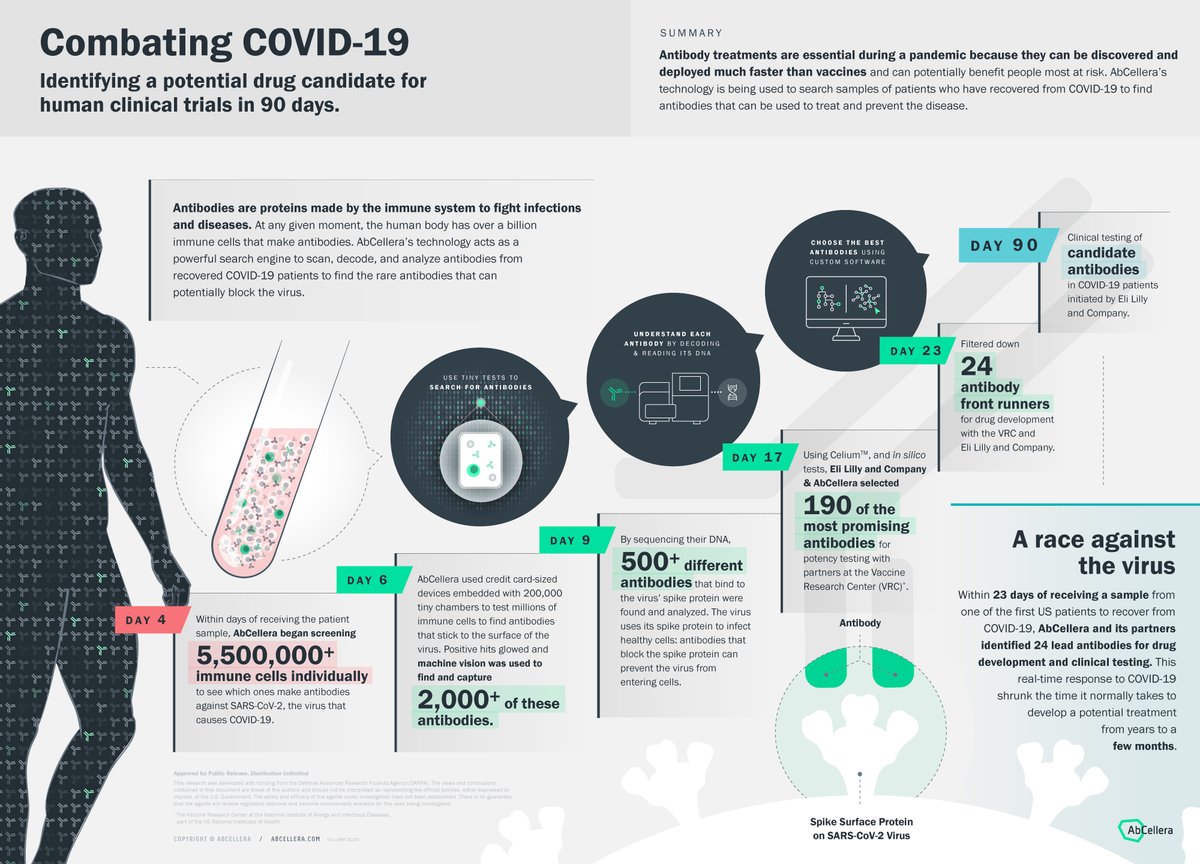 Abcellera Abcellerabio Twitter
Abcellera Abcellerabio Twitter
 Jasper Summit Research To Offer Covid 19 Clinical Trial Daily Mountain Eagle
Jasper Summit Research To Offer Covid 19 Clinical Trial Daily Mountain Eagle
 Understanding Clinical Decision Making During The Covid 19 Pandemic A Cross Sectional Worldwide Survey Eclinicalmedicine
Understanding Clinical Decision Making During The Covid 19 Pandemic A Cross Sectional Worldwide Survey Eclinicalmedicine
 Uchicago Medicine Begins Clinical Trial Testing The Efficacy Of Antibody Against Sars Cov 2 Uchicago Medicine
Uchicago Medicine Begins Clinical Trial Testing The Efficacy Of Antibody Against Sars Cov 2 Uchicago Medicine
Niaid Creates Clinical Trials Network To Focus On Covid 19 Vaccines Monoclonal Antibodies Biospace
 Clinical Trial Suggests Regeneron S Monoclonal Antibody Cocktail Prevents Covid 19 Clinical Daily News Mcknight S Long Term Care News
Clinical Trial Suggests Regeneron S Monoclonal Antibody Cocktail Prevents Covid 19 Clinical Daily News Mcknight S Long Term Care News
 Science Of Vaccines And Monoclonal Antibodies Covid 19 Prevention Network
Science Of Vaccines And Monoclonal Antibodies Covid 19 Prevention Network
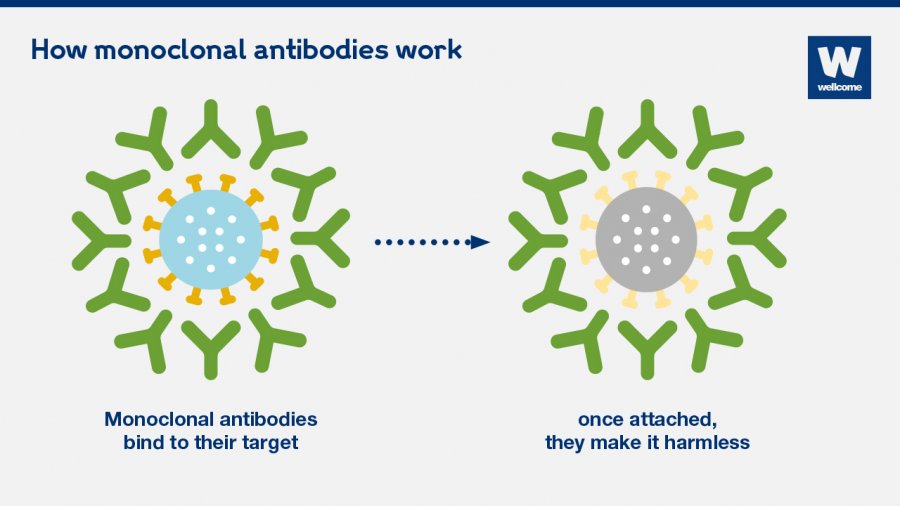 What Are Monoclonal Antibodies And Can They Treat Covid 19 Gavi The Vaccine Alliance
What Are Monoclonal Antibodies And Can They Treat Covid 19 Gavi The Vaccine Alliance
 A Strategic Approach To Covid 19 Vaccine R D Science
A Strategic Approach To Covid 19 Vaccine R D Science
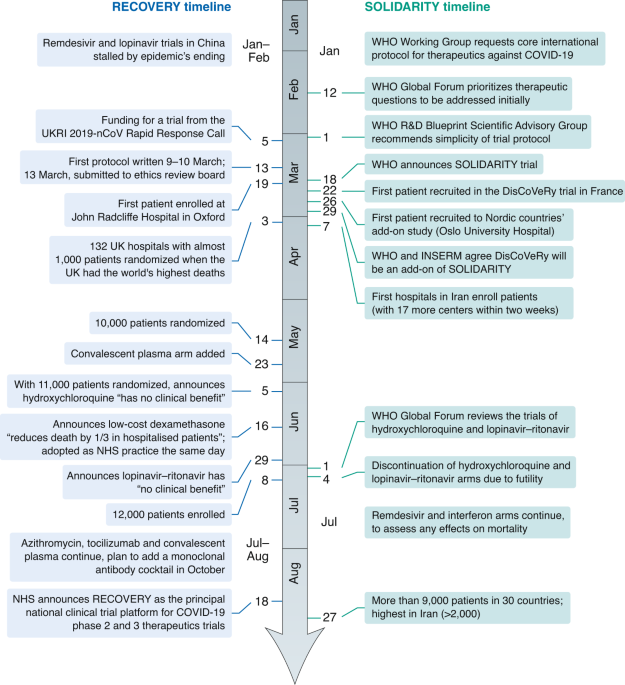 Covid 19 Clinical Trials Learning From Exceptions In The Research Chaos Nature Medicine
Covid 19 Clinical Trials Learning From Exceptions In The Research Chaos Nature Medicine
 Ongoing Clinical Trials For The Management Of The Covid 19 Pandemic Trends In Pharmacological Sciences
Ongoing Clinical Trials For The Management Of The Covid 19 Pandemic Trends In Pharmacological Sciences
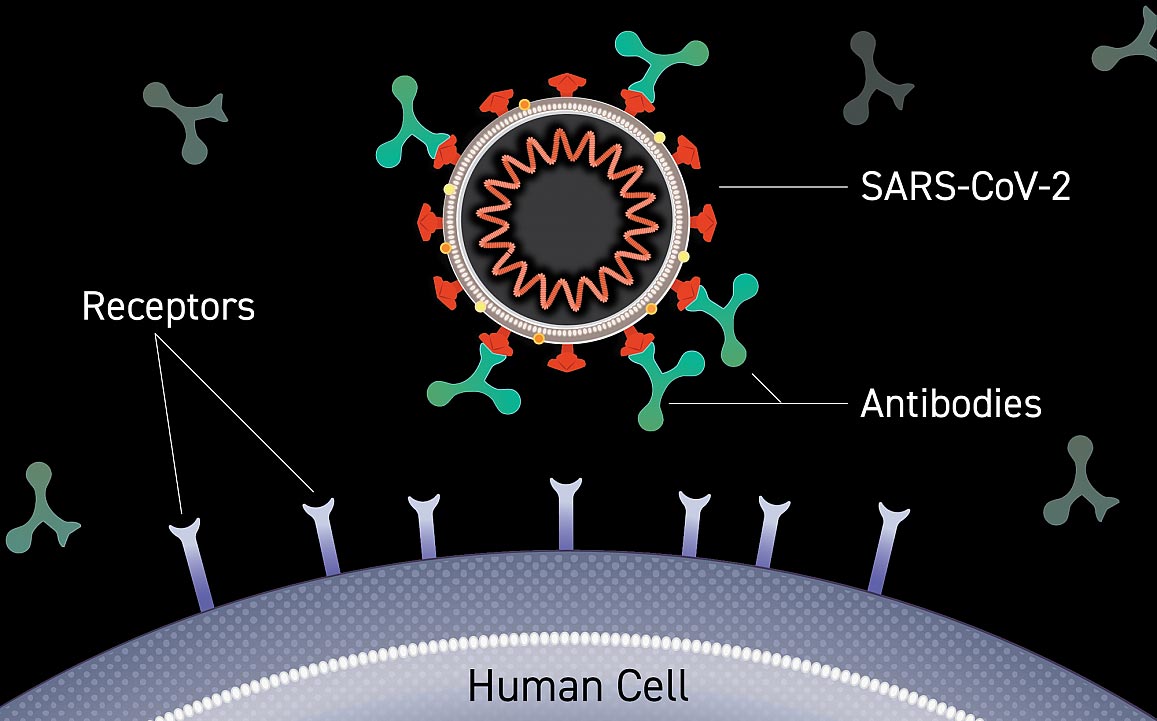 Monoclonal Antibodies To Prevent Covid 19 Phase 3 Clinical Trials Now Enrolling
Monoclonal Antibodies To Prevent Covid 19 Phase 3 Clinical Trials Now Enrolling
Https Sharedsystems Dhsoha State Or Us Dhsforms Served Le3515 Pdf
 Uic Opens Phase 3 Monoclonal Antibody Clinical Trial Uic Today
Uic Opens Phase 3 Monoclonal Antibody Clinical Trial Uic Today
Usc Enrolling For Phase 3 Clinical Trial To Test Covid 19 Monoclonal Antibody Treatment Keck School Of Medicine Of Usc
 Um Begins Testing Antibody Drug Regeneron To Prevent Covid Spread Inventum University Of Miami Miller School Of Medicine
Um Begins Testing Antibody Drug Regeneron To Prevent Covid Spread Inventum University Of Miami Miller School Of Medicine
 Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr
Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr
 Clinical Trials Nih Covid 19 Research
Clinical Trials Nih Covid 19 Research
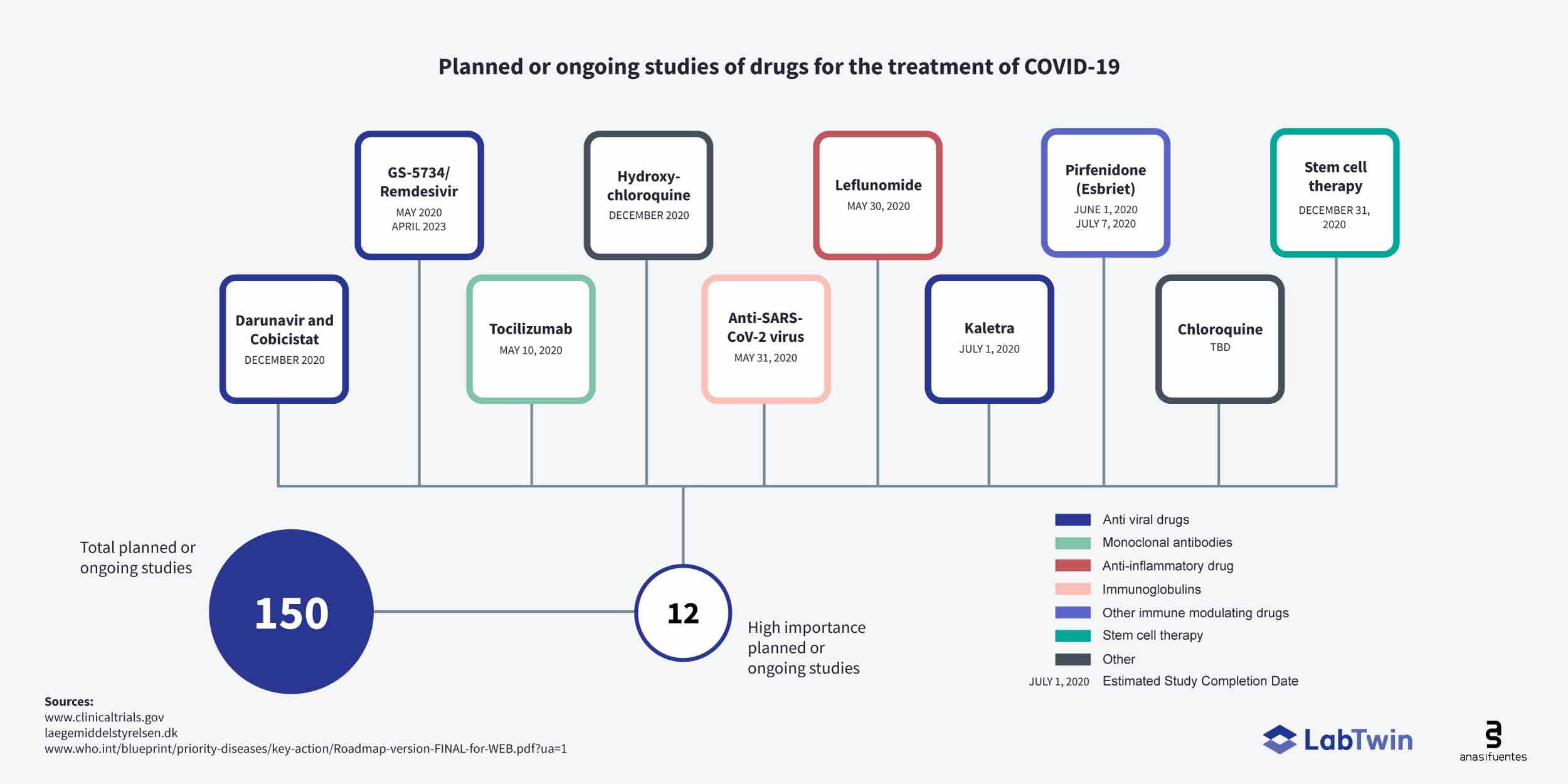
Post a Comment for "Covid Monoclonal Antibody Clinical Trials"