What Diagnosis Code Do You Use For Covid Antibody Testing
Presumptive positive COVID-19 test result. For pneumonia due to COVID -19 use U071 as the primary diagnosis and use J1282 as the secondary diagnosis Jan.
 Covid 19 Faqs Welcome To Pwnhealth
Covid 19 Faqs Welcome To Pwnhealth
Additionally the American Medical Association AMA Current Procedural Terminology CPT Editorial Panel has creat ed CPT code 87635 Infectious agent detection by nucleic acid DNA or RNA.
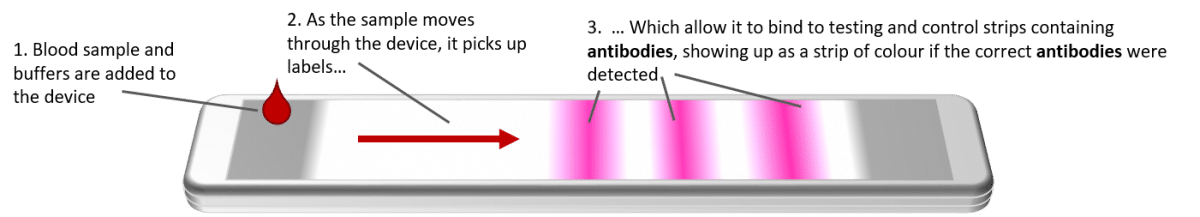
What diagnosis code do you use for covid antibody testing. The test provides a numerical value that indicates whether or not you may have antibodies to COVID-19. This test checks for antibodies to COVID-19. Diagnosis coding for COVID-19 is complicated in part because the World Health Organization WHO created two diagnosis codes for COVID-19 but so far the United States has adopted only one.
Separate Reimbursement for Preadmission COVID-19 Diagnostic Testing COVID-19 Diagnostic Test Specimen Collection Coding. However if the specimen will be prepared by your office and sent to an outside lab report the specimen collection code 99000. The providers documentation that the individual has COVID-19.
We have been told that the World Health Organization WHO has approved an emergency ICD-10 code of U072 COVID-19 virus not identified. The Interim Guidance for COVID-19 Antibody Testing in Clinical and Public Health Settings provides detailed information on how to make the best use of antibody tests. COVID-19 Diagnostic Test Coding Condition Code 51.
Use CPT code 99001 or 99211 where appropriate Commercial Use CPT codes 99000 andor 99001 C9803 Outpatient hospital facility only Telehealth Standard EM code Bill with appropriate EM code and one of the appropriate ICD -10 diagnosis codes. The American Medical Association Friday released two Current Procedural Terminology codes 86328 and 86769 for reporting antibody testing for the novel coronavirus and revised its CPT code for SARS-CoV-2 nucleic acid tests 86318. Confirmation does not require documentation of the type of test.
This is an exception to the hospital inpatient guideline Section II H. 87635 is designed to detect the COVID-19 virus and effective March 13 2020 and 86328 and 86769 will be used to identify the presence of antibodies to the COVID-19 virus and are effective April 10 2020. Presumptive positive COVID-19 test results should be coded as confirmed.
87635 Infectious agent detection by. 1 2021 and after. For example codes for exposure to COVID-19 or observation for suspected COVID-19 but where the tests are negative.
Antibody testing codes CPT codes 86328 and 86769 for COVID-19 antibody testing are effective and must be used for dates of service as of April 10 2020 and after. For a confirmed diagnosis assign code U071 COVID-19. Testing There is no code for swabbing the patient for COVID-19 much like there is no code for swabbing for influenza.
In addition two new PLA codes 0225U and 0226U were accepted for detection of. Severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 Coronavirus disease COVID-19 86408 Neutralizing antibody severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. The providers documentation that the individual has COVID-19 is sufficient.
People who receive positive results on an antibody test but dont have symptoms of COVID-19 and have not been around someone who may have COVID-19 are not likely to have a. For multisystem inflammatory syndrome use U071 as. Severe acute respiratory syndrome coronavirus 2 SARS -CoV-2 Coronavirus disease COVID -19 amplified probe technique Please visit http swwwama-.
Z03818 Z20828 or Z20822 COVID-19 laboratory testing billing guide. For a confirmed diagnosis assign code U071 COVID-19. The antibody test codes come on the heels of a new CPT code for COVID-19 diagnostic testing instituted last month.
86328 Immunoassay for infectious agent antibodyies qualitative or semi quantitative single step method eg reagent strip. In this context confirmation does not require documentation of the type of test performed. Follow CMS billing guidelines.
Assign code U071 COVID-19 for a confirmed diagnosis of the 2019 novel coronavirus disease COVID-19 as documented by the provider a positive COVID-19 test result or a presumptive positive COVID-19 test result. Sinai Laboratory is effective and must be used for dates of service as of June 25 2020 and after. The Centers for Medicare Medicaid Services CMS developed two new lab testing codes.
For confirmed COVID-19 use U071 as the primary diagnosis April 1 2020 and after. Code only a confirmed diagnosis of the 2019 novel coronavirus disease COVID-19 as documented by the provider documentation of a positive COVID-19 test result or a presumptive positive COVID-19 test result. Please note that all aforementioned changes are not included in CPT 2020 code set.
CPT code 0224U for SARS-CoV-2 COVID-19 antibody testing developed by Mt. For an encounter for COVID-19 testing being performed as part of preoperative testing assign code Z01812 Encounter for preprocedural laboratory examination as the first-listed diagnosis and assign code Z20828 or Z20822 depending on the encounter date as an additional diagnosis. Accepted addition of codes 86408 for reporting coronavirus 2 SARS-CoV-2 neutralizing antibody screen and 86409 for reporting coronavirus 2 SARS-CoV-2 neutralizing antibody titer.
There are three codes for COVID-19 testing. Confirmation does not require documentation of the type of test performed. If youve been exposed to COVID-19 or vaccinated your body produces antibodies as part of your immune response.
Providers can manually upload the code descriptors into their electronic health record systems.
 Covid 19 Information For Patients Sonora Quest
Covid 19 Information For Patients Sonora Quest
 Antibody Tests For Coronavirus Can Miss The Mark Npr
Antibody Tests For Coronavirus Can Miss The Mark Npr
 Coding For Covid 19 Testing Acog
Coding For Covid 19 Testing Acog
Https Www Premera Com Medicalpolicies 2 04 518 Pdf
Https Www Questdiagnostics Com Home Covid 19 Hcp Molecular And Serology Testing Information
 Coronavirus Antibody Testing In Seminole Fl Afc Urgent Care
Coronavirus Antibody Testing In Seminole Fl Afc Urgent Care
Covid 19 Testing Information Sheboygan County
Tri County Health Department Official Website
 Covid 19 And Antibody Testing What You Need To Know University Of Utah Health
Covid 19 And Antibody Testing What You Need To Know University Of Utah Health
 Coronavirus Antibody Tests Fda Raises Standards Shots Health News Npr
Coronavirus Antibody Tests Fda Raises Standards Shots Health News Npr
 What S The Difference Between Covid 19 Diagnostic And Antibody Tests
What S The Difference Between Covid 19 Diagnostic And Antibody Tests
 Covid 19 Antibody Active Infection Testing In San Diego County Fallbrook Regional Health District
Covid 19 Antibody Active Infection Testing In San Diego County Fallbrook Regional Health District
 What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
 What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
Https Www Un Org Sites Un2 Un Org Files Coronavirus Icd10codingguidance Pdf
 What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
What Tests Could Potentially Be Used For The Screening Diagnosis And Monitoring Of Covid 19 And What Are Their Advantages And Disadvantages The Centre For Evidence Based Medicine
 What Can Covid 19 Antibody Tests Really Tell Us Path
What Can Covid 19 Antibody Tests Really Tell Us Path
 Covid 19 What To Know About Antibody Testing Cedars Sinai
Covid 19 What To Know About Antibody Testing Cedars Sinai
 What You Need To Know About The Covid 19 Antibody Test Baltimore Sun
What You Need To Know About The Covid 19 Antibody Test Baltimore Sun
Post a Comment for "What Diagnosis Code Do You Use For Covid Antibody Testing"