Coronavirus Vaccine Johnson And Johnson Approval
Updated on March 16 2021 On February 27 Johnson Johnsons vaccine against the coronavirus disease 2019 COVID-19 became the third COVID-19 vaccine to receive emergency use authorization EUA from the US. The recommendation comes one day after the Food and Drug Administration on Saturday authorized Johnson Johnsons COVID-19 vaccine for emergency use.

US approves Johnson Johnson COVID-19 vaccine.
Coronavirus vaccine johnson and johnson approval. Johnson Johnson applies for emergency approval in US. The Advisory Committee on Immunization Practices ACIP will hold its second emergency meeting to discuss JJJanssen COVID-19 Vaccine on April. If approved public health experts say the Johnson JohnsonJanssen vaccine would be a game changer in the battle against coronavirus as it.
Food and Drug Administration FDA. Information about the Janssen COVID-19 Vaccine manufactured by Janssen Biotech Inc a Janssen Pharmaceutical Company of Johnson Johnson. The company said on Tuesday it had submitted an application to Indias drug regulator - the Drugs Controller General of India - requesting approval for the bridging safety and.
BENGALURU Reuters -Johnson Johnson is seeking to conduct a local clinical trial in India for its single-dose COVID-19 vaccine which was paused in the United States last week on reports of rare blood clots. Johnson Johnson coronavirus vaccine could receive FDA approval as early as next week Derek Chauvin is on trial for George Floyds death. Johnson Johnsons Janssen COVID-19 Vaccine.
The CDC and FDA have recommended a pause in the use of the Janssen Johnson Johnson COVID-19. Reuters - Johnson Johnsons one-dose COVID-19 vaccine appeared safe and effective in trials the US. Vaccine pictured here to allow.
A panel of vaccine advisers at the Food and Drug Administration will meet Friday to discuss whether the agency should grant emergency use authorization to the COVID-19 vaccine developed by Johnson. Johnson Johnson has become the latest company to request emergency authorization for its vaccine in the US hoping to. The vaccine is the third to become available.
Zients the White House Covid-19 response coordinator said Tuesday that pausing the use of Johnson Johnsons coronavirus vaccine. Food and Drug Administration said Wednesday paving the. A third coronavirus vaccine could soon be available in the United States a one-shot regimen made by pharmaceutical giant Johnson Johnson that proved safe and effective in.
Johnson Johnsons Covid-19 single-shot vaccine was shown to be 66 effective in preventing moderate and severe disease in a global Phase 3 trial the. The federal government on Friday is expected to lift its pause on use of the Johnson Johnson COVID-19 vaccine but there are signs that rare. CDC and FDA have recommended a pause in the use of Johnson Johnsons JJJanssen COVID-19 Vaccine in the United States out of an abundance of caution effective Tuesday April 13.
Johnson Johnsons one-shot COVID-19 vaccine received approval by US regulators.
 Company At Heart Of Johnson Johnson Vaccine Woes Emergent Biosolutions Has Series Of Citations Ktla
Company At Heart Of Johnson Johnson Vaccine Woes Emergent Biosolutions Has Series Of Citations Ktla
 Coronavirus Us Approves Johnson Johnson Covid 19 Vaccine News Dw 27 02 2021
Coronavirus Us Approves Johnson Johnson Covid 19 Vaccine News Dw 27 02 2021
 Johnson Johnson Covid 19 Vaccine Here S What To Know Abc News
Johnson Johnson Covid 19 Vaccine Here S What To Know Abc News
 Johnson Johnson Covid 19 Vaccine Here S What To Know Abc News
Johnson Johnson Covid 19 Vaccine Here S What To Know Abc News
 Johnson Johnson Vaccine Deliveries Plunge As Company Backs Off April Target The Washington Post
Johnson Johnson Vaccine Deliveries Plunge As Company Backs Off April Target The Washington Post

 Catholic Bishops Recommend Avoiding Johnson Johnson Shot If Possible Adding To Worries About Covid Vaccine Misgivings Colorado Public Radio
Catholic Bishops Recommend Avoiding Johnson Johnson Shot If Possible Adding To Worries About Covid Vaccine Misgivings Colorado Public Radio
 J J Says Batch Of Covid Vaccine Failed Quality Check Not Clear How Many Doses Were Lost Ktla
J J Says Batch Of Covid Vaccine Failed Quality Check Not Clear How Many Doses Were Lost Ktla
 The Johnson Johnson Vaccine Candidate For Covid 19 Explained
The Johnson Johnson Vaccine Candidate For Covid 19 Explained
 Single Shot Covid 19 Vaccine Trial Likely In India Soon Johnson Johnson In Touch With Centre
Single Shot Covid 19 Vaccine Trial Likely In India Soon Johnson Johnson In Touch With Centre
 Covid Vaccine J J Is 66 Effective But Single Shot May Fall Short Against Variants
Covid Vaccine J J Is 66 Effective But Single Shot May Fall Short Against Variants
 Johnson Johnson Vaccine Rolls Out Amid Concerns About Bias From Bad Publicity Npr
Johnson Johnson Vaccine Rolls Out Amid Concerns About Bias From Bad Publicity Npr
 J J Signs Covid 19 Vaccine Purchase Agreement With Avat
J J Signs Covid 19 Vaccine Purchase Agreement With Avat
 Johnson Johnson S Vaccine May Soon Be Approved Here S How It Works
Johnson Johnson S Vaccine May Soon Be Approved Here S How It Works
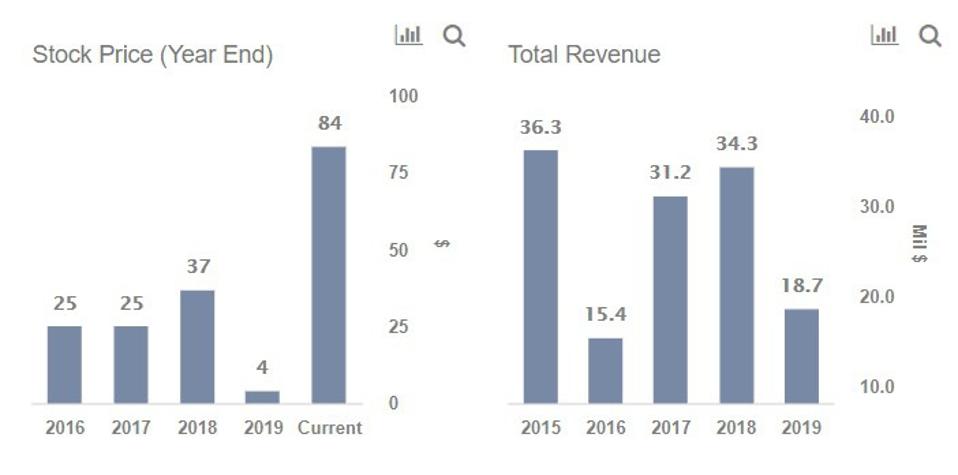 What S Happening With Johnson Johnson S Covid 19 Vaccine Rollout
What S Happening With Johnson Johnson S Covid 19 Vaccine Rollout
 Eu Commission Approves Johnson Johnson S One Shot Covid 19 Vaccine
Eu Commission Approves Johnson Johnson S One Shot Covid 19 Vaccine
 Johnson Johnson Covid 19 Vaccine Batch Fails Quality Check
Johnson Johnson Covid 19 Vaccine Batch Fails Quality Check
 Johnson Johnson S Covid 19 Vaccine Is Likely Hours Or Days Away From Authorization In The Us Abc7 San Francisco
Johnson Johnson S Covid 19 Vaccine Is Likely Hours Or Days Away From Authorization In The Us Abc7 San Francisco
 Johnson Johnson Applies For Emergency Use Authorization For Covid 19 Vaccine Coronavirus Updates Npr
Johnson Johnson Applies For Emergency Use Authorization For Covid 19 Vaccine Coronavirus Updates Npr
Post a Comment for "Coronavirus Vaccine Johnson And Johnson Approval"