Covid 19 Rapid Blood Test Kit
The sensitivity of COVID-19 IgGIgM Rapid Test Cassette Whole BloodSerumPlasma early after infection in unknown. The COVID-19 IgGIgM Rapid Test Cassette Whole BloodSerumPlasma is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM.
 Covid 19 Point Of Care Antibody Test 20 Test Kits Kahntact Medical
Covid 19 Point Of Care Antibody Test 20 Test Kits Kahntact Medical
There two main ways to test whether a person has COVID-19- Reverse Transcription Polymerase or RT-PCR and the Rapid Antibody Test to detect the presence of the virus.

Covid 19 rapid blood test kit. The Curative test is a simple-to-use 100 contactless oral fluid COVID-19 test that can be rapidly scaled to enable widespread access to testing to keep our communities and employees safe. It can take at least two. SVY Blood Screening Market to Reach 356 Billion by 2027 Growing at a CAGR of 69 From 2020 With COVID-19 Impact -.
The test is self-administered painless and has clinical accuracy of 90 and 100 specificity. The Assure COVID-19 IgGIgM Rapid Test Device is a rapid lateral flow chromatographic immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human venous whole blood sodium EDTA serum plasma sodium EDTA and fingerstick whole blood. Rapid tests are point-of-care diagnostic tests that use a mucus sample from the nose or throat but can be analyzed at a.
The reason why India has opted for surveillance over mass testing is because of the shortage of testing kits available. Michael Dao he acquired the tests from South Korea and will be using them at his office in Westminster CA. Our COVID-19 IgGIgM Test cassette is for qualitative detection of IgGIgM antibodies to SARS-CoV-2 in human body it just needs 10ul serum plasma or whole blood read.
These tests look for the presence of antibodies which are proteins made in response to infections. The test kit has been validated at the companys international partner facility and with US-based laboratories in New York and California. This type of testing is valuable because it can identify those who may have been asymptomatic and recovered.
CDC has developed interim guidance for how healthcare providers laboratories and public health staff should use antibody tests. What is a rapid COVID-19 test. All it tells you is whether youve been infected at some point in the past even if that occurred months ago.
Antibodies are detected in the blood of people who are tested after infection. FDA Director General Eric Domingo said they have approved the use of 5 brands of rapid test kits. PHILADELPHIA PA ACCESSWIRE May 5 2020 Avidium Labs in partnership with NXT Group has introduced a rapid IgGIgM antibody test kit for SARS-CoV-2 also known as COVID-19.
Health Covid-19 virus Subject. A COVID-19 antibody test cannot diagnose active coronavirus infection. How does testing work.
The test provides a numerical value that indicates whether or not you may have antibodies to COVID-19. Serology tests for COVID-19 Serology testing for SARS-CoV-2 continues to be in high demand because it can help to better quantify the total number of cases of COVID-19 to date. Today that EUA is being reissued to authorize the test for POC use using fingerstick blood samples.
The Assure COVID-19 IgGIgM Rapid Test Device was first authorized for emergency use by certain labs in July 2020 to help identify individuals with antibodies to SARS-CoV-2 indicating recent or prior COVID-19 infection. The Rapid COVID-19 IgMIgG Combo Test Kit is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2 indicating recent or prior infection. Testing was performed at two sites in China from January to mid-March 2020.
The statement said. Covid-19 rapid test includes a test card once-time use lancet and solution. Negative results do not preclude acute SARS-CoV-2 infection False positive results for COVID-19 IgGIgM Rapid Test Cassette Whole BloodSerumPlasma may occur due to cross-reactivity from pre-existing antibodies or other.
The clinical performance of the COVID-19 IgGIgM Rapid Test Cassette Whole BloodSerumPlasma was evaluated by testing a total of 191 plasma K2EDTA clinical samples90 positive samples and 101 negative samples from individual patients exhibiting pneumonia respiratory symptoms and fever etc. If you have been exposed to the virus that causes COVID-19 your body typically produces antibodies as part of the immune response to the virus. MANILA The Food and Drug Administration FDA announced on Monday that it has approved COVID-19 rapid test kits despite the Department of Healths preference for real-time polymerase chain reaction RT-PCR based test kits.
They show the bodys efforts to fight off a specific infection.
 Azure Biotech Covid 19 Rapid Igg Igm Antibody Test Ecotest Stat Technologies
Azure Biotech Covid 19 Rapid Igg Igm Antibody Test Ecotest Stat Technologies
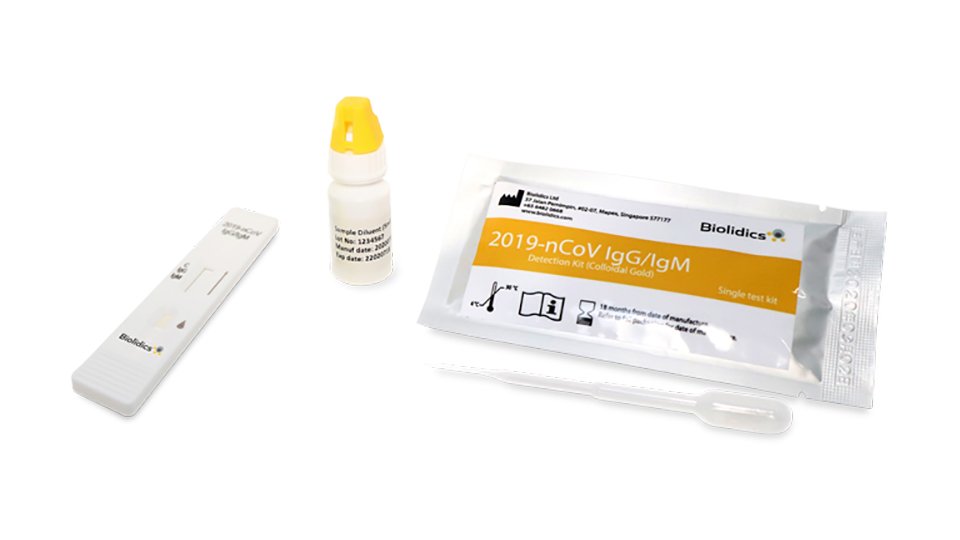 Biolidics Launches Rapid Test Kit For Covid 19
Biolidics Launches Rapid Test Kit For Covid 19
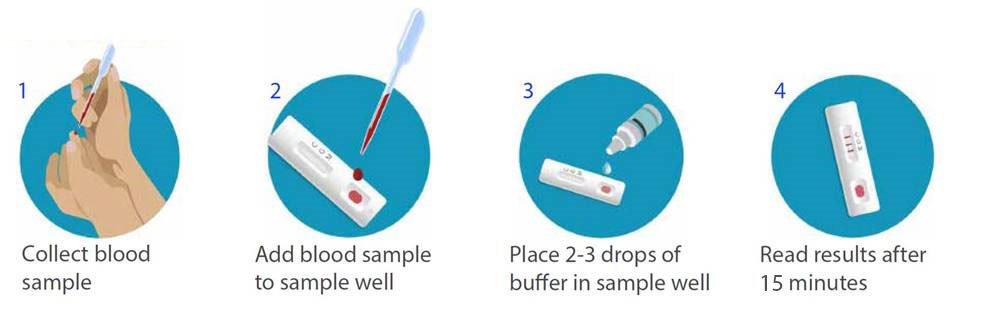 Bd Biomedomics Announce Launch Of Rapid Serology Test To Detect Exposure To Covid 19
Bd Biomedomics Announce Launch Of Rapid Serology Test To Detect Exposure To Covid 19
 South Korean Ivd Company Sugentech S Covid 19 Igm Igg Rapid Test Listed On Fda
South Korean Ivd Company Sugentech S Covid 19 Igm Igg Rapid Test Listed On Fda
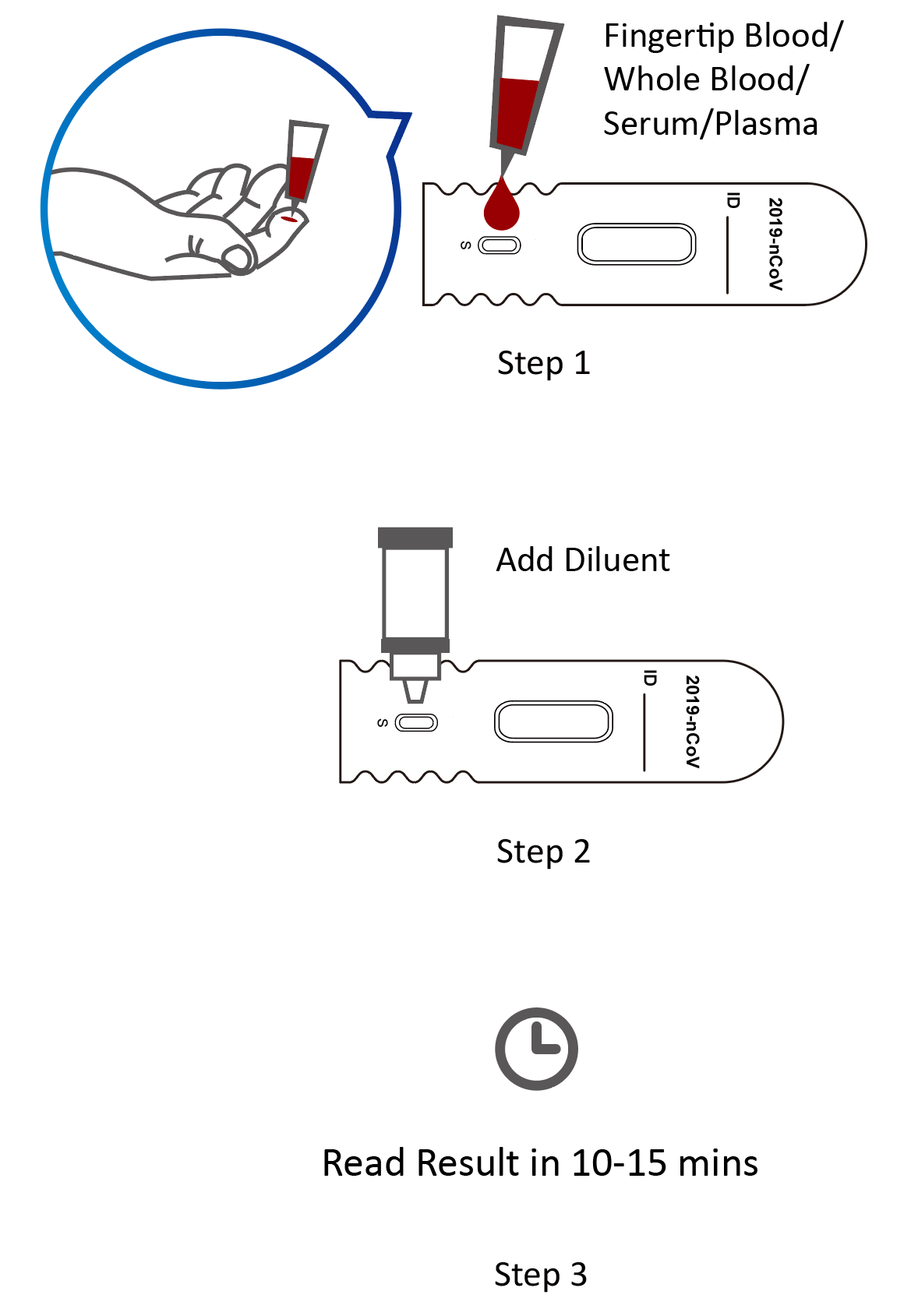 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
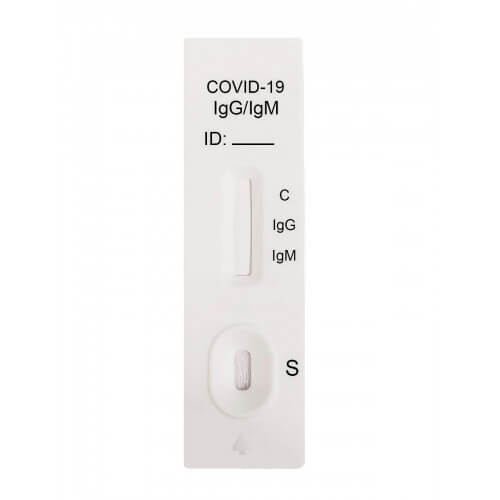 Sars Cov 2 Igg Igm Rapid Test Kit Abbexa Ltd
Sars Cov 2 Igg Igm Rapid Test Kit Abbexa Ltd
 Biomedomics Teams With Bd To Launch Rapid Covid 19 Test
Biomedomics Teams With Bd To Launch Rapid Covid 19 Test
 Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
 Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
 Covid 19 Igg Igm Rapid Test Cassette Jant Pharmacal Corporation
Covid 19 Igg Igm Rapid Test Cassette Jant Pharmacal Corporation
 Biolidics Announces Plans To Launch Its Covid 19 Rapid Test Kits In The Us Mobihealthnews
Biolidics Announces Plans To Launch Its Covid 19 Rapid Test Kits In The Us Mobihealthnews
 Coronavirus Covid 19 Igg Igm Rapid Test Kit 25 Tests Per Box
Coronavirus Covid 19 Igg Igm Rapid Test Kit 25 Tests Per Box
 Coronavirus Covid 19 Igg Igm Rapid Test Kit 25 Tests Per Box
Coronavirus Covid 19 Igg Igm Rapid Test Kit 25 Tests Per Box
 Local Firm Develops Rapid Test Kit That Can Detect Covid 19 In Less Than 10 Minutes Today
Local Firm Develops Rapid Test Kit That Can Detect Covid 19 In Less Than 10 Minutes Today


Post a Comment for "Covid 19 Rapid Blood Test Kit"