What Is Nucleic Acid Amplification Test (naat) For Covid-19
In-Clinic Testing Diagnostic. In-Home Test Kit Receive your kit in two business days.
 Nucleic Acid Amplification Tests On Respiratory Samples For The Diagnosis Of Coronavirus Infections A Systematic Review And Meta Analysis Clinical Microbiology And Infection
Nucleic Acid Amplification Tests On Respiratory Samples For The Diagnosis Of Coronavirus Infections A Systematic Review And Meta Analysis Clinical Microbiology And Infection
The molecular test for SARS-CoV-2 COVID-19 from Quest Diagnostics is a reverse Nucleic Acid Amplification Test NAAT test that looks for the presence of viral RNA in a respiratory specimen.
What is nucleic acid amplification test (naat) for covid-19. All COVID-2019 SARS-CoV-2 nucleic acid amplificationmolecular tests NAAT and antigen test results positive and non-positive must be reported electronically within one working day. COVID Ready Testing management. Virtual Consultation Talk to a.
FDA issues an EUA for the Cue COVID-19 Test for Home and OTC Use a molecular nucleic acid amplification test and the first molecular test authorized for OTC without a prescription. Individuals complete an upper respiratory self-collection for this test and specimens are sent to a Quest Diagnostics lab for processing. It is reportedly quicker and the machines for the test.
Laboratories should report only the results from serologic tests that have an FDA Emergency Use Authorization EUA. A nucleic acid test analyzes tiny amounts of DNA or RNA in a sample of blood tissue or body fluid. Because the amount of genetic material is very small the test may include a step where the DNA or RNA of the microorganism is amplified or increased.
COVID-19 Travel Clearance COVID-19 clearance tests. The CBNAAT COVID-19 test is alternate Cartridge-based nucleic acid amplification test CBNAAT. COVID-19 Vaccination Book your COVID-19 vaccine visit or join the waitlist.
This type of nucleic acid pathogen test is known as a nucleic acid amplification test or NAAT.
 Covid 19 Device Testing Why Your Study Will Probably Need Full Board Review Pearl Irb
Covid 19 Device Testing Why Your Study Will Probably Need Full Board Review Pearl Irb
 Comparison Of Different Samples For 2019 Novel Coronavirus Detection By Nucleic Acid Amplification Tests International Journal Of Infectious Diseases
Comparison Of Different Samples For 2019 Novel Coronavirus Detection By Nucleic Acid Amplification Tests International Journal Of Infectious Diseases
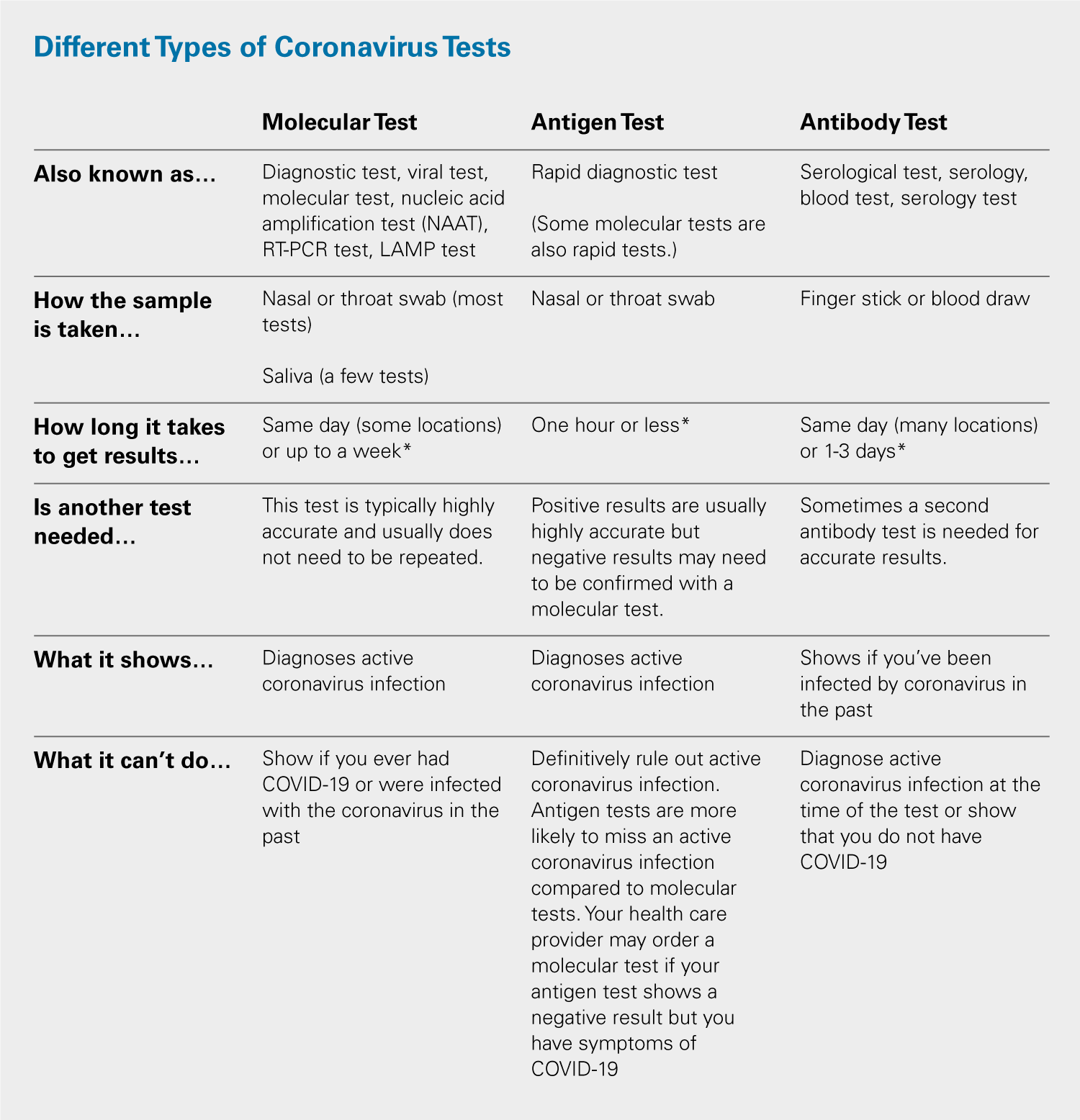
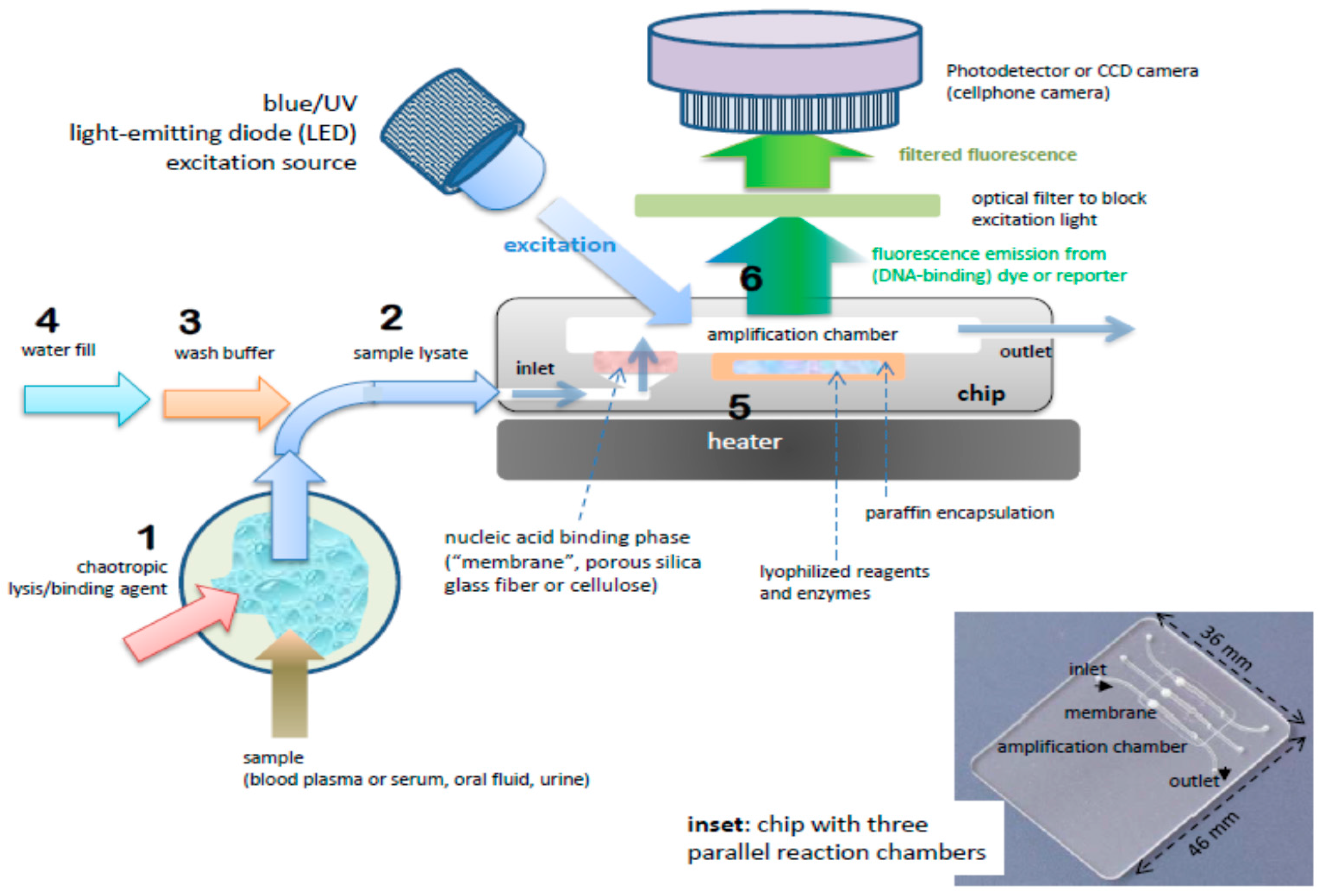 Biosensors Free Full Text Simple Approaches To Minimally Instrumented Microfluidic Based Point Of Care Nucleic Acid Amplification Tests Html
Biosensors Free Full Text Simple Approaches To Minimally Instrumented Microfluidic Based Point Of Care Nucleic Acid Amplification Tests Html
 Developing A Sound Strategy For Testing Screening Your Employees For Covid 19 Blue Cross Blue Shield
Developing A Sound Strategy For Testing Screening Your Employees For Covid 19 Blue Cross Blue Shield
 Poster 1 Seth Ack Diagnostic Capabilities Of Nucleic Acid Amplification Testing For Covid 19 Office Of Undergraduate Research
Poster 1 Seth Ack Diagnostic Capabilities Of Nucleic Acid Amplification Testing For Covid 19 Office Of Undergraduate Research
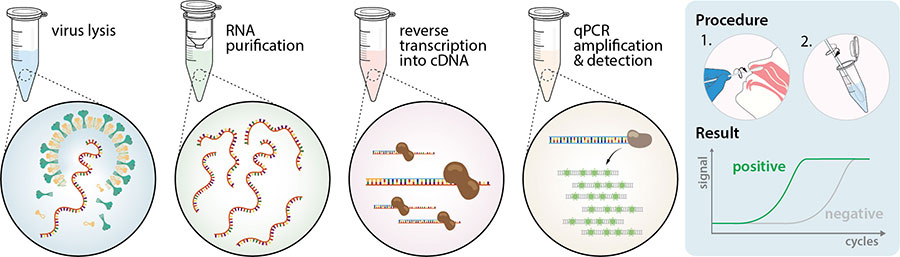 How Do Coronavirus Disease Covid 19 Tests Work Enzo Life Sciences
How Do Coronavirus Disease Covid 19 Tests Work Enzo Life Sciences
 Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine
Simpler And Faster Covid 19 Testing Strategies To Streamline Sars Cov 2 Molecular Assays Ebiomedicine
 Testing For Sars Cov 2 Covid 19 A Systematic Review And Clinical Guide To Molecular And Serological In Vitro Diagnostic Assays Reproductive Biomedicine Online
Testing For Sars Cov 2 Covid 19 A Systematic Review And Clinical Guide To Molecular And Serological In Vitro Diagnostic Assays Reproductive Biomedicine Online
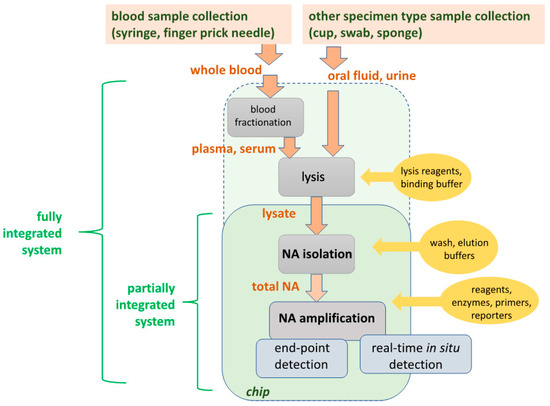 Biosensors Free Full Text Simple Approaches To Minimally Instrumented Microfluidic Based Point Of Care Nucleic Acid Amplification Tests Html
Biosensors Free Full Text Simple Approaches To Minimally Instrumented Microfluidic Based Point Of Care Nucleic Acid Amplification Tests Html
Types Of Covid 19 Tests Winchester Ma Official Website
Covid 19 Testing Information Sheboygan County
 Covid 19 Testing For Employees Employers Quest Diagnostics
Covid 19 Testing For Employees Employers Quest Diagnostics
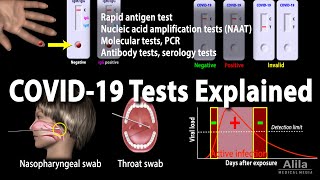 Understanding Different Covid 19 Tests Animation Youtube
Understanding Different Covid 19 Tests Animation Youtube
 Understanding Covid 19 Testing Tallahassee Memorial Healthcare Tallahassee Fl
Understanding Covid 19 Testing Tallahassee Memorial Healthcare Tallahassee Fl
Covid 19 Testing How It Works And Why We Need It Urgently Thoughts From The Centre Deloitte Uk
 Covid 19 Testing A One Page Basic Guide
Covid 19 Testing A One Page Basic Guide
 Implementation Of Antigen Rdt Ag Rdt To Detect Covid 19 Cases In Indonesia
Implementation Of Antigen Rdt Ag Rdt To Detect Covid 19 Cases In Indonesia
 How Do Coronavirus Disease Covid 19 Tests Work Enzo Life Sciences
How Do Coronavirus Disease Covid 19 Tests Work Enzo Life Sciences
Post a Comment for "What Is Nucleic Acid Amplification Test (naat) For Covid-19"