Covid Antibody Igg - Elisa Range
Please use one of the following formats to cite this article in your essay paper or report. IgM levels increased during the first week after.
 Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Please note it may take 14-21 days to produce detectable levels of IgG following infection.

Covid antibody igg - elisa range. To reduce the likelihood of a false-positive result the CDC Interim Guidelines for COVID-19 Antibody Testing suggest using an. The mean concentration of SARS-CoV-2-IgG-antibodies of the positive 5 outpatients was lower than in symptomatic patients with COVID-19 n 12 and positive PCR of SARS-CoV-2 304 258 versus 805 670. This is a semi-quantitative ELISA-based test for COVID-19-associated antibodies.
IgM and IgG antibody levels were assessed via chemiluminescence immunoassay in 338 COVID-19 patients. A COVID-19 antibody test also known as a serology test is a blood test that can detect if a person has antibodies to SARS-CoV-2 the virus that causes COVID-19. Overview of ELISA Testing for COVID-19 Antibodies.
SARS-CoV-2-specific serum-IgG antibodies in severe and mild COVID-19. There are no current recommendations for assessing COVID-19 vaccine response. 4 of 5 patients had elevated SARS-CoV-2-IgA.
This test has a sensitivity of 100 meaning the test will currently identify COVID-19 IgG antibody if it is present in the blood 100 of the time and a specificity of 998 meaning the test will correctly determine that there are no antibodies in the blood nearly all the time. Samples which are contaminated microbiologically should not be used. Three hundred fifty-four blood samples longitudinally obtained from 81 IgG-seroconverting progressed coronavirus disease 2019 CoVID-19 patients were quantified for spike 1 S1 S2.
The patients with severe symptoms were older mean age 58 and all male compared to patients with mild symptoms mean. Based on data the tests developers provided to. Forty-seven patients provided a total of 156 serum samples mean 33 per patient range 17 5117 days PSO.
Food and Drug Administration has so far authorized just eight for emergency use. Use for the detection of IgG antibodies against the spike protein S1 of SARS-CoV-2 COVID-19 that develop in response to natural infection with SARS-CoV-2 or from a COVID-19 vaccination. The antibody instant COVID-19 test contains a conjugate pad with SARS-CoV-2 recombinant antigens an IgG line coated with an anti-human IgM line an IgM line coated with anti-human IgM and a control line.
In this study we comprehensively analyzed multispecific antibody kinetics of different immunoglobulins in hospitalized patients with acute severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 infection. If you had symptoms consistent with COVID-19 within the past 3 weeks and tested negative repeat testing in 1-2 weeks may yield a positive result. After the sample is placed inside the test cassette the specimen will migrate by capillary action along with the cassette.
Reactive Positive results may be due to past or present infection with SARS-CoV-2. Any patient samples used in manufacturing have been heat inactivated prior to handling. If any antibodies are present they will bind to.
SARS-CoV-2 antibodies particularly IgG antibodies may persist for months and possibly years. This means you have not been infected with COVID-19. The COVID-19 Self-Collected Antibody Test System is an enzyme-linked immunosorbent assay ELISA and a blood collection kit intended for qualitative detection of IgG antibodies to SARS-.
Positive results for spike IgG are quantified by serial dilution titer. This test detects IgG antibodies against the SARS-CoV-2 spike antigen and can be used to infer past infection or vaccination. Of the 47 patients 1547 32 had severe and 3247 68 had mild COVID-19.
This study aimed to determine the IgM and IgG responses against severe acute respiratory syndrome coronavirus SARS-CoV-2 in coronavirus disease 2019 COVID-19 patients with varying illness severities. COVID-19 antibody tests can help. These data on kinetics of SARS-CoV-2 antibodies are consistent with reports showing patients with COVID-19 in general with detectable IgG and IgM in plasma between four and 7 days POS21 To strengthen the study we compared the antibody titre measurements by ELISA to those obtained on a commercially available instrument the Ortho VITROS total.
Though coronavirus antibody tests have flooded the market the US. The Anti-SARS-CoV-2 COVID-19 IgG AccuBind ELISA Test System is a qualitative assay and does not necessarily give an indication of quantities of IgG antibodies. The kit developed in a months time would help to study SARS CoV-2 IgG antibodies presence in the Indian population.
The SARS-CoV-2 IgG assay is a qualitative test designed to detect IgG antibodies to the nucleocapsid protein of SARS-CoV-2 in serum and plasma from patients who are suspected of past coronavirus disease COVID-19 or in serum and plasma of subjects that may have been infected by SARS-CoV-2. On National Technology Day ICMRDELHI is proud to announce the development of first indigenous Human IgG ELISA kit for Covid_19 testing. Serum IgG antibodies against SARS-CoV-2 were significantly higher in COVID-19 case patients median 201 units interquartile range 016-4433 units than in all persons in the control groups median 010 unit interquartile range 005-019 unit.
You tested negative for COVID-19 IgG antibody. A positive antibody test can help support a diagnosis when patients present with complications of COVID-19 illness such as multisystem inflammatory syndrome and other post-acute sequelae of COVID-19. Reference range of anti-SARS-CoV-2-IgA and IgG was defined as ratio for negative 08 borderline 08-11 and 11 positive.
 Analytical And Clinical Performances Of Five Immunoassays For The Detection Of Sars Cov 2 Antibodies In Comparison With Neutralization Activity Ebiomedicine
Analytical And Clinical Performances Of Five Immunoassays For The Detection Of Sars Cov 2 Antibodies In Comparison With Neutralization Activity Ebiomedicine
 Covid 19 N Protein Human Igg Elisa Kit Ab274339 Abcam
Covid 19 N Protein Human Igg Elisa Kit Ab274339 Abcam
 Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
 Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
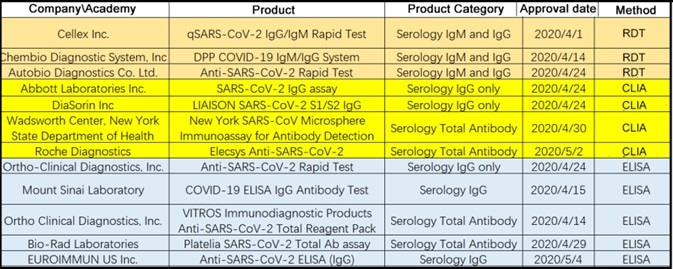 Overview Of Elisa Testing For Covid 19 Antibodies
Overview Of Elisa Testing For Covid 19 Antibodies
Http Www Sah Org Ar Pdf Covid 19 Nr4sapkd4prgtfwhz Pdf
New Insights Into Serological Coronavirus Testing Westburg
 Covid 19 Elisa Kit Human Covid 19 Spike Protein S1 Igg Igm Coronavirus Elisa Kit
Covid 19 Elisa Kit Human Covid 19 Spike Protein S1 Igg Igm Coronavirus Elisa Kit
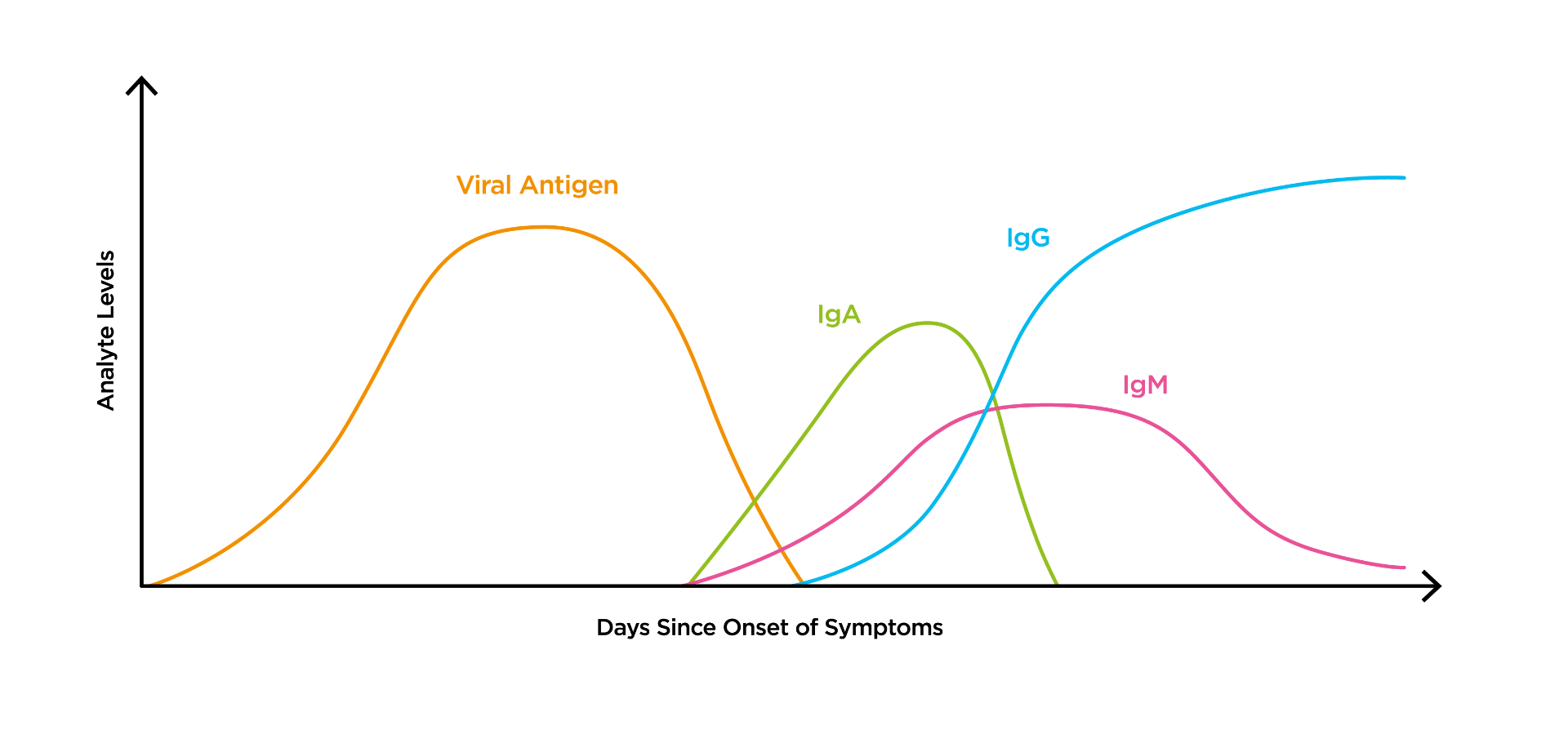 Coronavirus Sars Cov 2 Covid 19 Igg Elisa
Coronavirus Sars Cov 2 Covid 19 Igg Elisa
 Overview Of Elisa Testing For Covid 19 Antibodies
Overview Of Elisa Testing For Covid 19 Antibodies
 Diagnostic Performance Of Seven Rapid Igg Igm Antibody Tests And The Euroimmun Iga Igg Elisa In Covid 19 Patients Clinical Microbiology And Infection
Diagnostic Performance Of Seven Rapid Igg Igm Antibody Tests And The Euroimmun Iga Igg Elisa In Covid 19 Patients Clinical Microbiology And Infection
 Performance Evaluation Of Five Elisa Kits For Detecting Anti Sars Cov 2 Igg Antibodies International Journal Of Infectious Diseases
Performance Evaluation Of Five Elisa Kits For Detecting Anti Sars Cov 2 Igg Antibodies International Journal Of Infectious Diseases
 Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
 Covid 19 Antibody Igg Elisa Machaon Diagnostics
Covid 19 Antibody Igg Elisa Machaon Diagnostics
 Re Evaluating Positive Serum Samples For Sars Cov 2 Specific Iga And Igg Antibodies Using An In House Serological Assay Clinical Microbiology And Infection
Re Evaluating Positive Serum Samples For Sars Cov 2 Specific Iga And Igg Antibodies Using An In House Serological Assay Clinical Microbiology And Infection
 Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
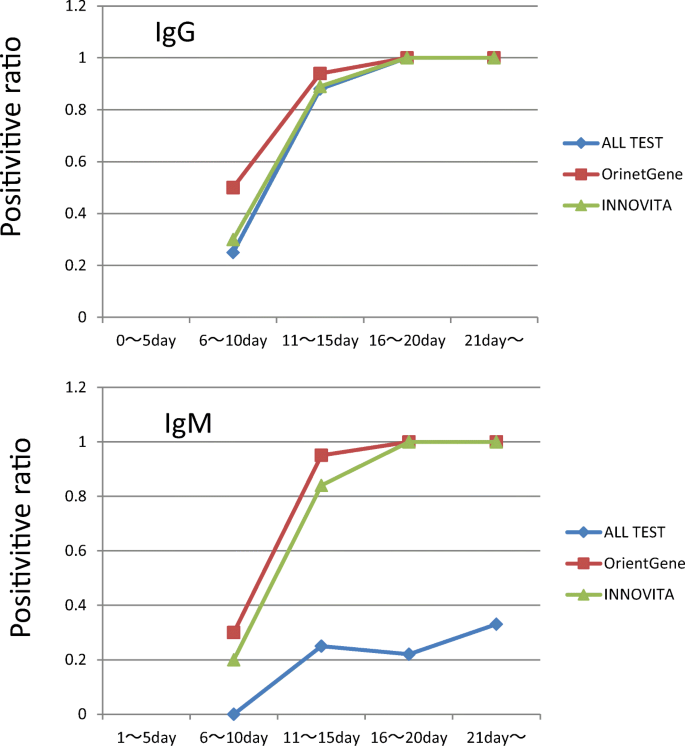 Investigation Of Anti Sars Cov 2 Igg And Igm Antibodies In The Patients With Covid 19 By Three Different Elisa Test Kits Springerlink
Investigation Of Anti Sars Cov 2 Igg And Igm Antibodies In The Patients With Covid 19 By Three Different Elisa Test Kits Springerlink
 Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
Covid Seroindex Kantaro Sars Cov 2 Igg Antibody Ruo Kit Dsr200 R D Systems
 Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Post a Comment for "Covid Antibody Igg - Elisa Range"