Coronavirus Antibody Test Kit Canada
This page provides resources to learn about COVID antibody. An antibody serology test tells if you have antibodies to the SARS-CoV-2 virus.
Sales Of Winnipeg Company S Covid 19 Detection Tests Halted By Health Canada Cbc News
The performance of authorized COVID-19 testing devices has not been assessed in people who are vaccinated against COVID-19.
Coronavirus antibody test kit canada. The term sensitivity is used instead of PPA for ease of reading. In Ontario and Manitoba the COVID-19 SARS-CoV-2 Antibody Test is 75. Labcorp will bill the cost of the COVID-19 antibody test directly to your health plan if you are insured or if you are uninsured Labcorp will bill the appropriate government program.
Get Tested 109 The Everlywell COVID-19 Test Home Collection Kit allows qualifying individuals to collect a sample and send it to a certified lab to receive digital results within 24-48 hours. To log in to the survey complete the following steps. By testing the blood of more than 10000 Canadians for antibodies which are developed after a person is exposed to the virus to fight it off the Action to Beat Coronavirus study is hoping to not.
Health Canada has published a resource on Information for patients on COVID-19 antibody serology testing. Audacia Bioscience co-CEO Phillip Olla said his company has developed a small. The table below includes applications that are under evaluation.
Windsor company seeks approval for COVID-19 antibodies test kit Health Canada says rapid blood test for COVID-19 remains under review Rapid antibody blood tests are being made in Canada but Health. Currently Dynacare offers this test in Ontario Manitoba and Quebec only. Serological testing detects the presence of antibodies not the presence of the SARS-CoV-2 virus.
Tests for viral infection and antibody serology tests. This Test Kit comes uses a nasal secretion sample and contains 25 tests. Is seeking approval from Health Canada for a rapid COVID-19 testing kit.
This testing is also not to be used to diagnose infection. For this reason the accuracy of a positive test result is expressed as a positive percent agreement PPA. Antibodies develop within days or weeks of your illness and linger in your system for a few months we dont know exactly how long yet afterward.
A positive result indicates that an individual has been exposed to the virus and mounted an immune response. Specific and sensitive antibody tests are needed to inform patient treatment and to aid in COVID-19 population surveillance. When you get sick with COVID your body produces antibodies.
Ontario has ordered over 900000 test kits while Alberta Health Services has signed a 95-million contract for 250 handheld devices along with 100000 test kits. Immune system cells that fight off the infectionAn antibody test detects the presence of these cells. Serological tests for COVID-19 are used to detect the presence of antibodies against SARS-CoV-2.
This page is updated daily by 500 am EST. A manufacturing company in Windsor Ont. For more information on this test in Quebec please contact Customer Care at 5144868025.
The cost of the test is 4213 and is based on rates established by the Centers for Medicare Medicaid Services CMS. A test for viral infection detects the virus or a component of the virus and tells you if you have a current COVID-19 infection. Statistics Canada is mailing out test kits to tens of thousands of people to study the prevalence of coronavirus in the country in the first survey of its.
Everlywell offers this test and collection kit with an FDA Emergency Use Authorization EUA. COVID-19 testing device applications authorized by Health Canada Currently there is no standard reference panel used globally. Enter your secure access code you will find this code in the invitation letter or email you previously received from Statistics Canada.
A COVID-19 antibody test looks for signs of a previous infection. This is done using a swab from your nose or throat or a saliva sample. Visit the electronic questionnaire portal and select Start my survey.
However Health Canada does not expect intramuscular COVID-19 vaccinations to interfere with the performance of authorized nucleic-acid or antigen-based testing devices. The COVID-19 Antigen Rapid Test Device is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasal and nasopharyngeal secretions. Two kinds of tests are currently available for COVID-19.
Statistics Canada is mailing test kits to 48000 Canadians in order to determine the presence of coronavirus antibodies in Canadians. Health Canada confirms that authorized COVID-19 tests are well supported by evidence indicating they will provide accurate and reliable results. Purchase Health Canada approved rapid result COVID-19 Test Kits.
 White Rock Man Among First To Receive Covid Antibody Test Says Completing It A Civic Duty Surrey Now Leader
White Rock Man Among First To Receive Covid Antibody Test Says Completing It A Civic Duty Surrey Now Leader
 Free Samples Wesail Biotech Covid Rapid Tests
Free Samples Wesail Biotech Covid Rapid Tests
 Health Canada Approved Covid 19 Antigen Antibody Rapid Test
Health Canada Approved Covid 19 Antigen Antibody Rapid Test
Questcap Arranges Secured Promissory Note To Finance Purchase Of Covid 19 Antibody Testing Kits Aequitas Neo Exchange Medv Ne
 Bc Company Receives Over 600k To Create Coronavirus Antibody Test News
Bc Company Receives Over 600k To Create Coronavirus Antibody Test News
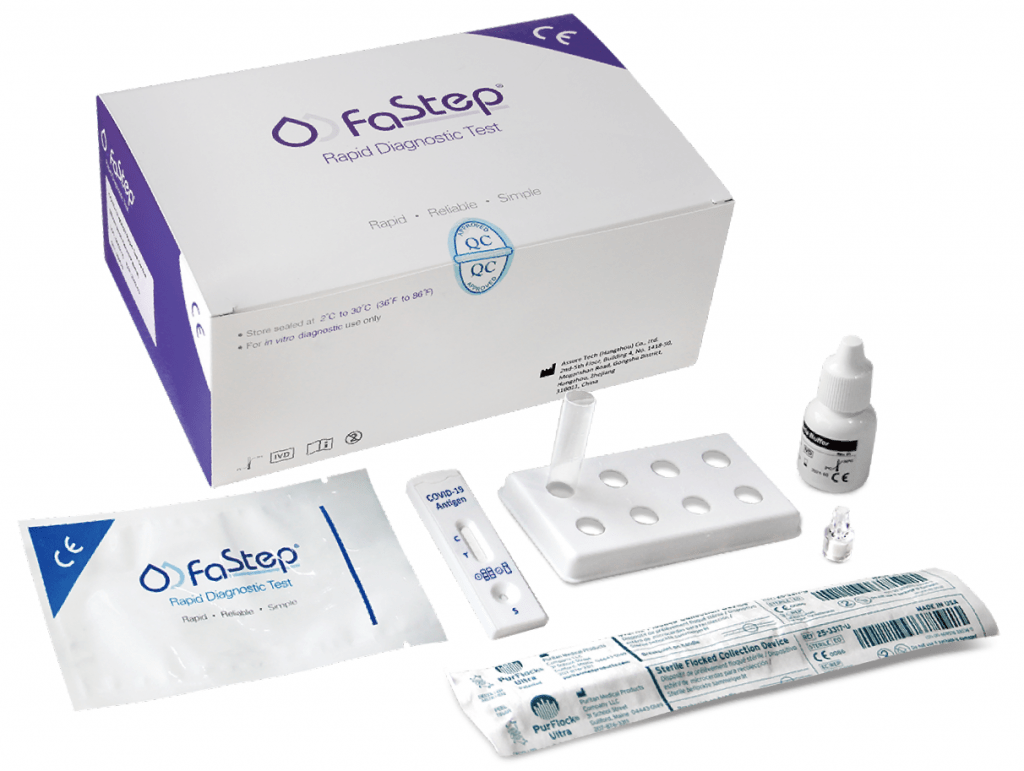 Health Canada Approved Covid 19 Antigen Antibody Rapid Test
Health Canada Approved Covid 19 Antigen Antibody Rapid Test
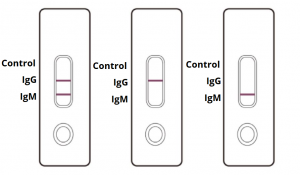 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
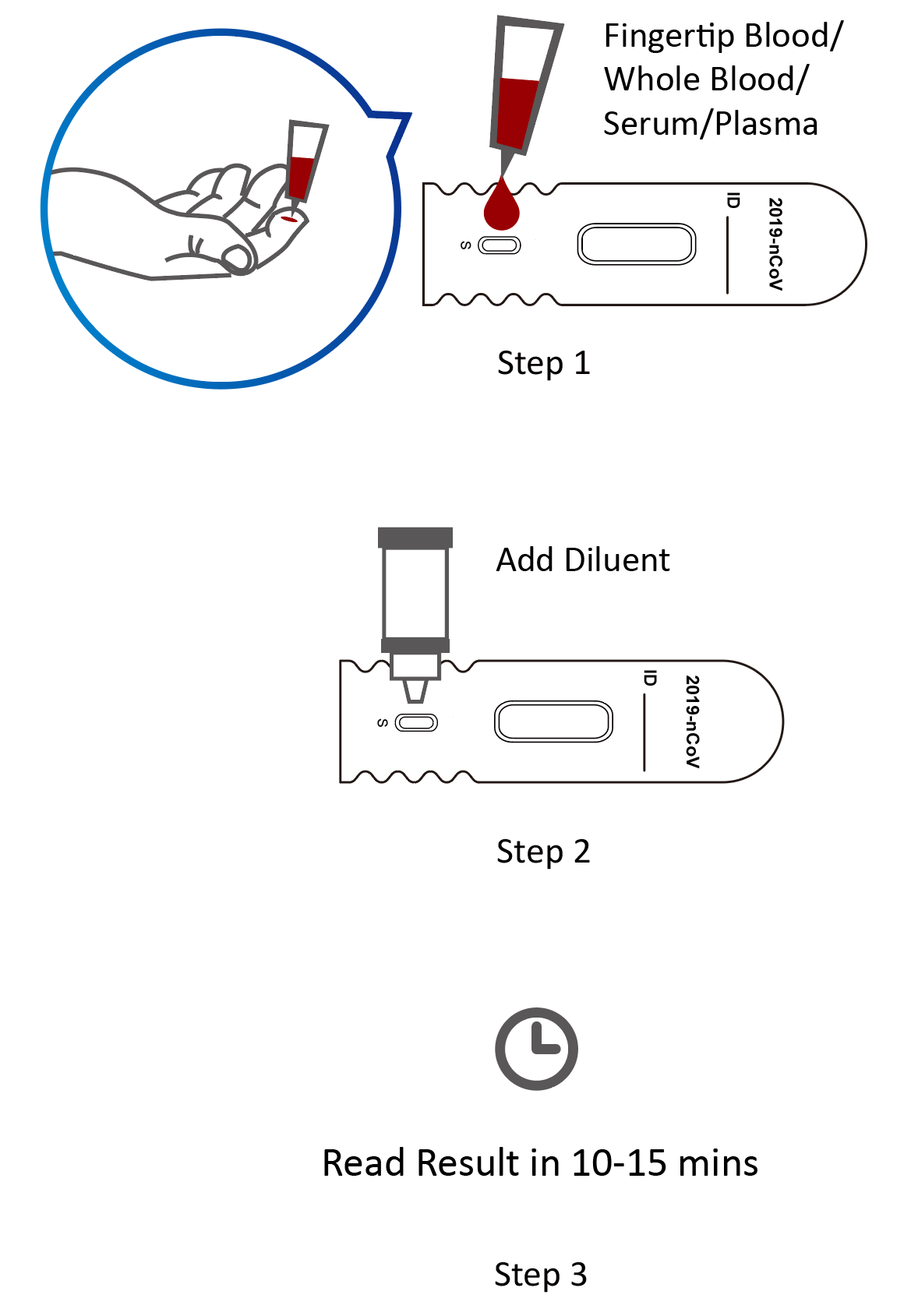 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Covid 19 Rapid Antibody Test Canadian Group Announces Results Of The Latest Independent Performance Evaluation Of Their Swiss Canadian Zekmed Rapid Antibody Test Head To Head With The Cdc Fda Serology Reference
Covid 19 Rapid Antibody Test Canadian Group Announces Results Of The Latest Independent Performance Evaluation Of Their Swiss Canadian Zekmed Rapid Antibody Test Head To Head With The Cdc Fda Serology Reference
 Biohit Sars Cov 2 Igm Igg Antibody Test Kit
Biohit Sars Cov 2 Igm Igg Antibody Test Kit
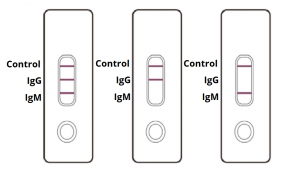 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Thousands Of Manitobans To Receive Covid 19 Antibody Survey Kit Cbc News
Thousands Of Manitobans To Receive Covid 19 Antibody Survey Kit Cbc News
 Health Canada Authorizes Canada S First Rapid Covid 19 Antibody Test
Health Canada Authorizes Canada S First Rapid Covid 19 Antibody Test
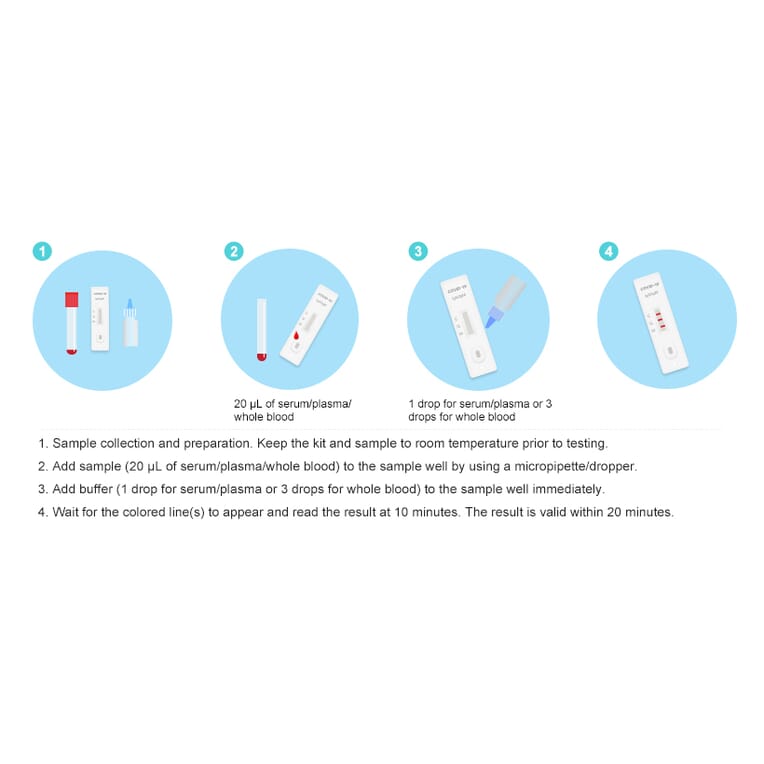

Post a Comment for "Coronavirus Antibody Test Kit Canada"