Covid Antibody Test Igg Or Total
Results from antibody testing should not be used as the sole basis to diagnose or exclude COVID-19 SARS-CoV-2 infection or to inform infection status. After the sample is placed inside the test cassette the specimen will migrate by capillary action along with the cassette.
 Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future
Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future
A negative test means that you have no COVID-19 antibodies so you probably were not infected with the COVID-19 virus in the past.

Covid antibody test igg or total. If you had symptoms consistent with COVID-19 within the past 3 weeks and tested negative repeat testing in 1-2 weeks may yield a positive result. IgM antibodies which happen early in an infection IgG antibodies which are. This test detects IgG antibodies against the SARS-CoV-2 spike antigen and can be used to infer past infection or vaccination.
To test this hypothesis we examined the ability of S1- and RBD-specific immunoglobulin G IgG from hospitalized and nonhospitalized COVID-19 patients and controls Table S1 to elicit ADCD ADCP and ADCC. False-positive test results can occur. And two test for total antibodies IgM IgG and IgA.
This test detects the presence of high affinity antibodies to the SARS-CoV-2 virus nucleocapsid protein. Four weeks after the second shot. In a review of 54 available studies through the end of April mostly from China the accuracy of pooled results for combination IgGIgM tests was 914 95 CI 870 - 966 for 15 to 21 days post-symptom onset.
Here we hypothesized that differential qualitative features of SARS-CoV-2-specific antibodies contribute to COVID-19 severity. IgG antibody to the COVID-19 vaccines. Quantitative detection of IgM and IgG antibodies against SARS-CoV-2 quantitatively has potential significance for evaluating the severity and prognosis of COVID-19.
The cost of the test is 4213 and is based on rates established by the Centers for Medicare Medicaid Services CMS. Qualitative detection of total antibodies including IgM IgA and IgG to SARS-CoV-2 in human serum run manually or using the Dynex AGILITY automated ELISA workstation. For Antibody Tests to be used in COVID-19 testing the World Health Organization recommends a minimum sensitivity and specificity of 98.
Four of the tests were designed to look for IgG antibodies only. Thus antibody tests provide a promise and a peril in the ongoing Covid-19 pandemic. In the event that your health plan or.
You tested negative for COVID-19 IgG antibody. Please note it may take 14-21 days to produce detectable levels of IgG following infection. The antibody instant COVID-19 test contains a conjugate pad with SARS-CoV-2 recombinant antigens an IgG line coated with an anti-human IgM line an IgM line coated with anti-human IgM and a control line.
1282021 When would a patients IgG antibody to the COVID-19 vaccines peak. The new SARS-CoV-2 Antibody IgG Spike Semi-Quantitative test result is reported as positive at an index 12 of 100. For Rapid Antibody Test Kits to be procured by the DOH HTAC recommends a minimum sensitivity and specificity of 98 for specimens collected 20 days or more after the appearance of first symptoms.
This means you have not been infected with COVID-19. One tests for both IgM and IgG. The test looks for one or both kinds of antibodies to SARS-CoV-2 the virus that causes COVID-19.
Because it takes time for antibodies to develop false-negative test. This positive result indicates that an individual has developed an. This test cannot distinguish between past infection versus vaccination.
A COVID-19 antibody test also known as a serology test is a blood test that can detect if a person has antibodies to SARS-CoV-2 the virus that causes COVID-19. COVID-19 antibody tests can help. It may be that the test detected antibodies to a coronavirus closely related to the COVID-19 virus or that the test quality was flawed.
Labcorp will bill the cost of the COVID-19 antibody test directly to your health plan if you are insured or if you are uninsured Labcorp will bill the appropriate government program. Detection of IgM and IgG antibodies in patients with coronavirus disease 2019. These data on kinetics of SARS-CoV-2 antibodies are consistent with reports showing patients with COVID-19 in general with detectable IgG and IgM in plasma between four and 7 days POS21 To strengthen the study we compared the antibody titre measurements by ELISA to those obtained on a commercially available instrument the Ortho VITROS total.
When checking vaccine response to Pneumovax we usually wait four to six weeks but since the current COVID-19 vaccines are a two-shot series when would the patients IgG peak. Antibody IgG SARS-CoV-2 Quantitative Total Antibody Test Number 164068 164055 164090 CPT Code 86769 86769 TBD Intended Use Qualitative detection of anti-SARS-CoV-2 antibodies. The SARS-CoV-2 IgG assay is a qualitative test designed to detect IgG antibodies to the nucleocapsid protein of SARS-CoV-2 in serum and plasma from patients who are suspected of past coronavirus disease COVID-19 or in serum and plasma of subjects that may have been infected by SARS-CoV-2.
Qualitative detection of IgG antibodies to SARS-CoV-2 the virus that causes COVID-19. The seventh test produced by Euroimmun is an enzyme immunoassay which uses enzymes to detect when antibodies in the blood bind to recombinant SARS-CoV-2 antigens.
 Validation And Performance Comparison Of Three Sars Cov 2 Antibody Assays Biorxiv
Validation And Performance Comparison Of Three Sars Cov 2 Antibody Assays Biorxiv
New Insights Into Serological Coronavirus Testing Westburg
 Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
 Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
 Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
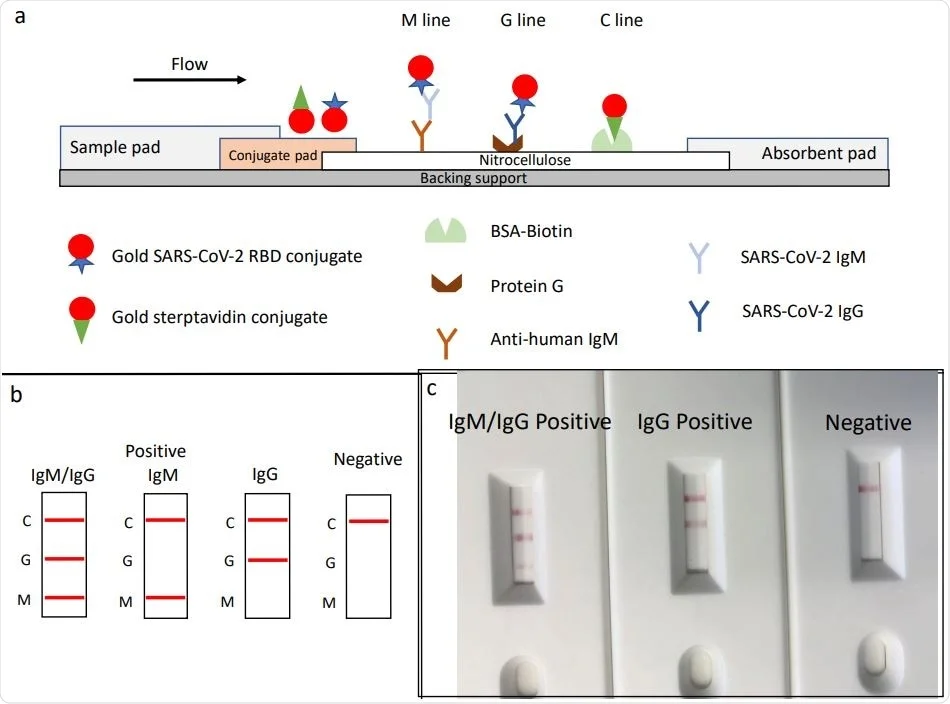 Rapid Sars Cov 2 Igm Igg Combined Antibody Test
Rapid Sars Cov 2 Igm Igg Combined Antibody Test
 Covid 19 Antibody Igg Igm Elisa Panel Machaon Diagnostics
Covid 19 Antibody Igg Igm Elisa Panel Machaon Diagnostics
 Seroprevalence Of Sars Cov 2 Immunoglobulin Antibodies In Wuhan China Part Of The City Wide Massive Testing Campaign Clinical Microbiology And Infection
Seroprevalence Of Sars Cov 2 Immunoglobulin Antibodies In Wuhan China Part Of The City Wide Massive Testing Campaign Clinical Microbiology And Infection
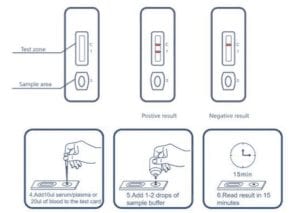 Coronavirus Covid 19 Antibody Test Glenbio
Coronavirus Covid 19 Antibody Test Glenbio
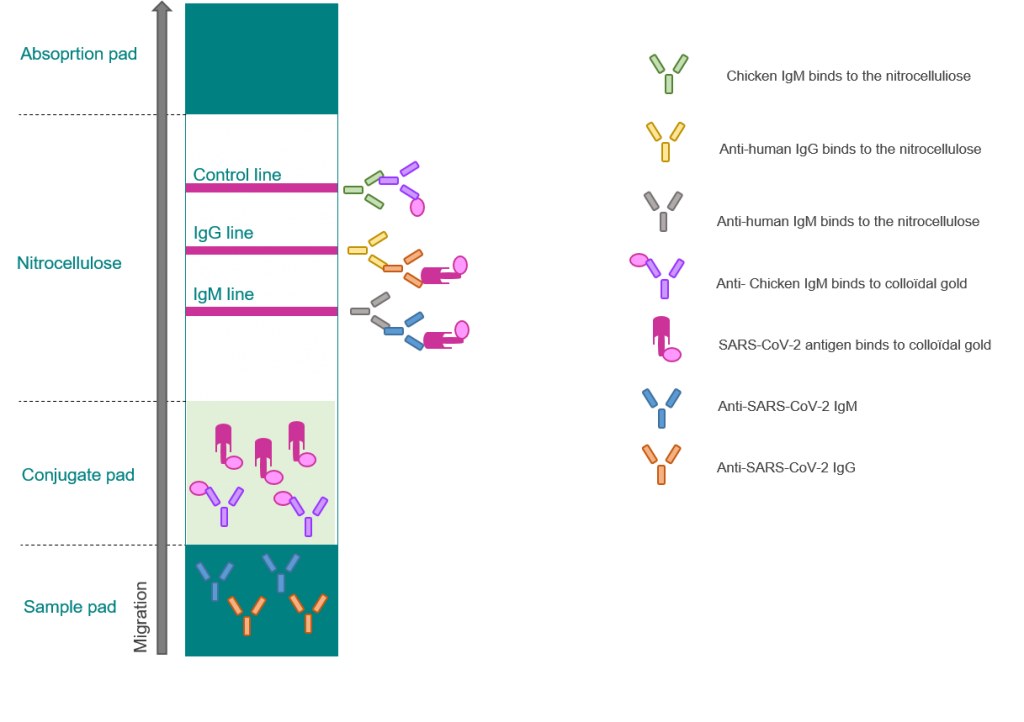 Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
Sars Cov 2 Covid 19 Diagnosis By Igg Igm Rapid Test Clinisciences
 Diagnostic Performance Of Seven Rapid Igg Igm Antibody Tests And The Euroimmun Iga Igg Elisa In Covid 19 Patients Clinical Microbiology And Infection
Diagnostic Performance Of Seven Rapid Igg Igm Antibody Tests And The Euroimmun Iga Igg Elisa In Covid 19 Patients Clinical Microbiology And Infection
 Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
Antibody Response Against Sars Cov 2 Spike Protein And Nucleoprotein Evaluated By Four Automated Immunoassays And Three Elisas Clinical Microbiology And Infection
 Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future
Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future
 Understanding Antibody Testing For Covid 19 The Journal Of Arthroplasty
Understanding Antibody Testing For Covid 19 The Journal Of Arthroplasty
 Sars Cov 2 Igg Antibody Test Receives Fda Emergency Use Authorization
Sars Cov 2 Igg Antibody Test Receives Fda Emergency Use Authorization
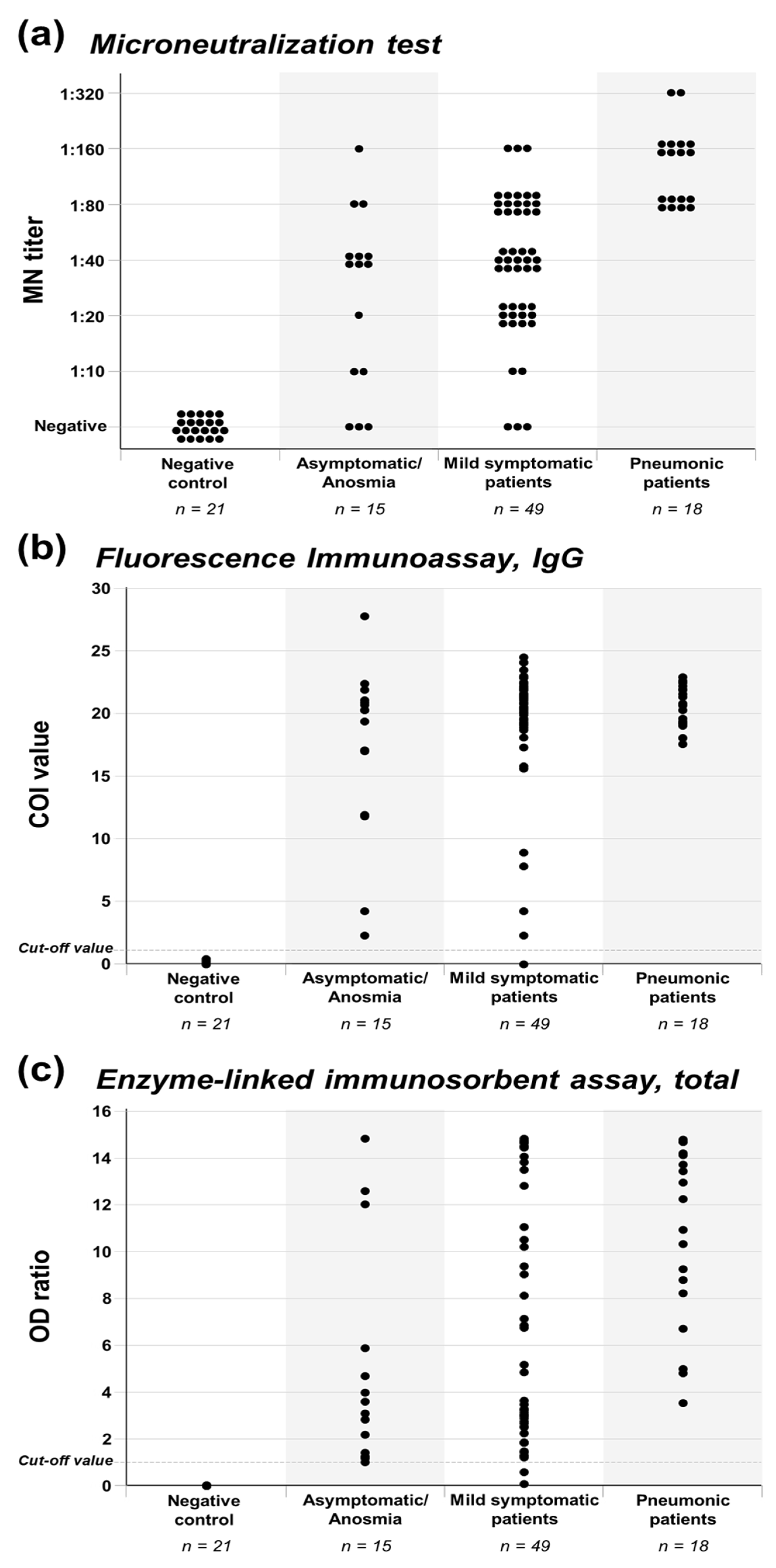 Jcm Free Full Text Neutralizing Antibody Production In Asymptomatic And Mild Covid 19 Patients In Comparison With Pneumonic Covid 19 Patients Html
Jcm Free Full Text Neutralizing Antibody Production In Asymptomatic And Mild Covid 19 Patients In Comparison With Pneumonic Covid 19 Patients Html
 Testing For Sars Cov 2 Covid 19 A Systematic Review And Clinical Guide To Molecular And Serological In Vitro Diagnostic Assays Reproductive Biomedicine Online
Testing For Sars Cov 2 Covid 19 A Systematic Review And Clinical Guide To Molecular And Serological In Vitro Diagnostic Assays Reproductive Biomedicine Online
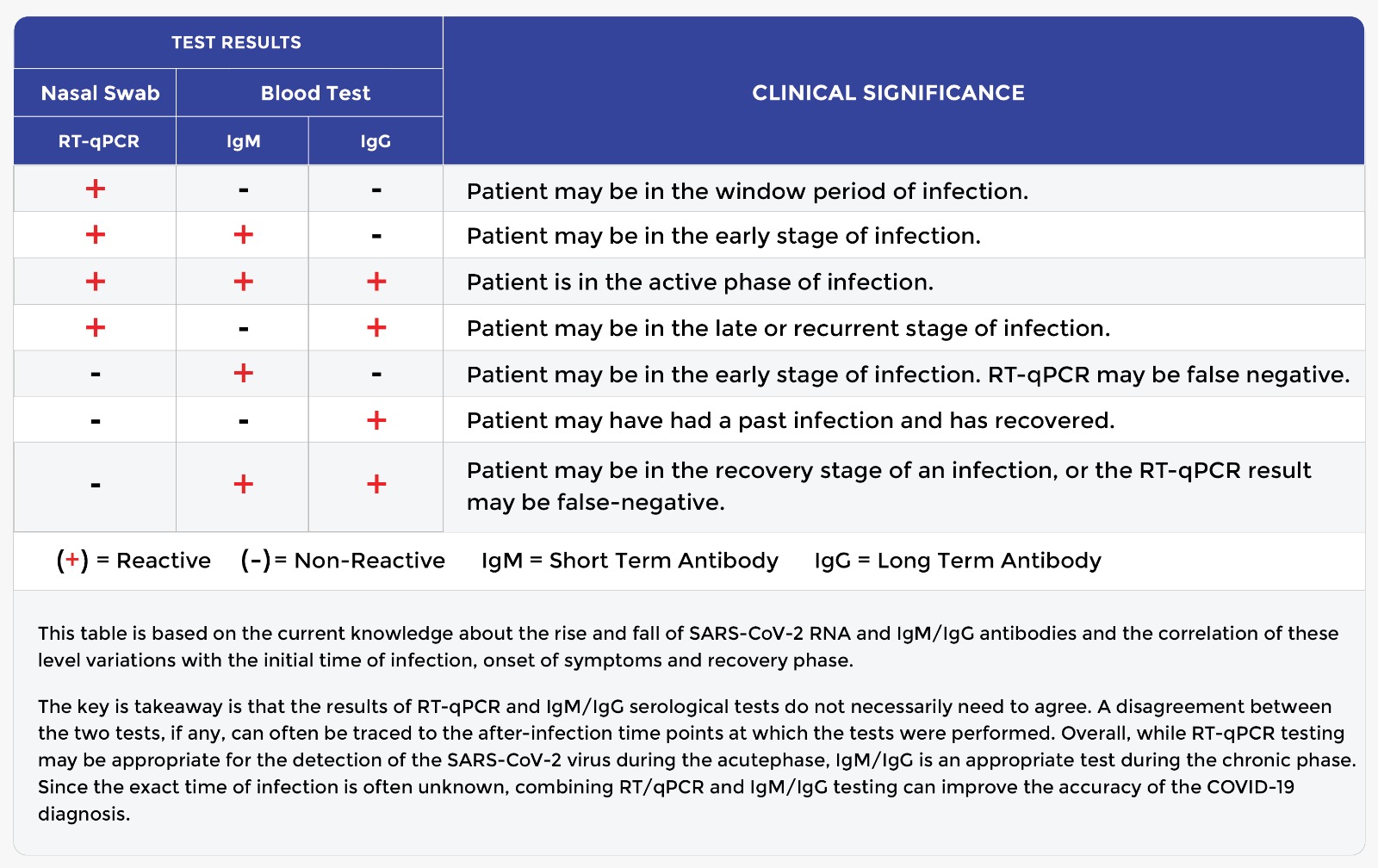 Covid 19 Antibody Testing Endocrinology Consultants P C
Covid 19 Antibody Testing Endocrinology Consultants P C
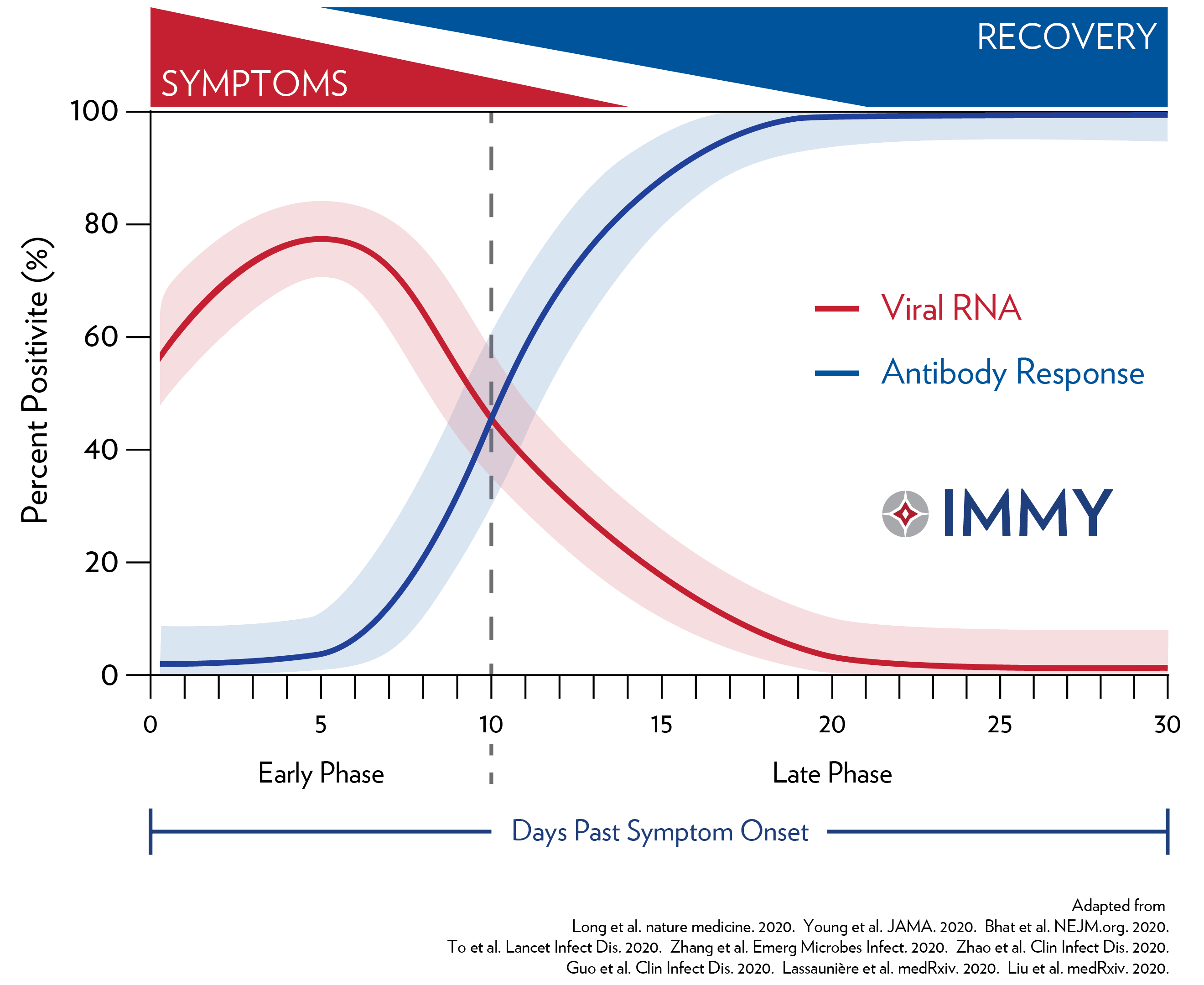
Post a Comment for "Covid Antibody Test Igg Or Total"