Coronavirus Antibody Treatment Eli Lilly
The FDAs authorization has allowed providers to use 700 milligrams of bamlanivimab to treat COVID-19 patients. Eli Lillys COVID-19 neutralizing antibody drug was the first of its kind to score FDA authorization against the illness in early November.
March 26 2021 The US.

Coronavirus antibody treatment eli lilly. LLY announced today they have entered into an agreement to co-develop antibody products for the treatment. US revokes authorisation of one of Eli Lillys COVID-19 monoclonal antibody treatments. The Food and Drug Administration has granted an emergency use authorization for Eli Lillys Covid-19 monoclonal antibody treatment called bamlanivimab.
AbCellera and Lilly will select from 500 unique antibodies isolated from one of the first US. The approval of the treatment which is manufactured by the drug maker Eli Lilly gives doctors another option for patients with Covid-19 who are. Emerging coronavirus variants later threatened to.
The US Food and Drug Administration FDA said on Friday that it has revoked it emergency authorisation of Eli Lillys COVID-19 monoclonal antibody treatment. Regulators to revoke their emergency authorization for the use of bamlanivimab alone. Eli Lilly and Co.
The announcement about bamlanivimab came Wednesday from the. INDIANAPOLIS Drugmaker Eli Lilly says its COVID-19 antibody drug should no longer be given to patients alone because treatment combinations work better fighting some variants of the coronavirus. Eli Lillys combination therapy of two antibodies bamlanivimab and etesevimab helped cut the risk of hospitalization and death in COVID-19 patients by.
The FDA action specifies that the therapy. Government has stopped the use of Eli Lillys COVID-19 monoclonal antibody treatments due to the spread of COVID-19 variants. After lab testing found Eli Lillys solo COVID-19 antibody couldnt match its combo against emerging coronavirus variants the feds stopped using it in.
Patients who recovered from COVID-19 to create antibody therapeutics for treatment and prevention of COVID-19. April 20 2021 - Eli Lilly and Company recently requested that FDA revoke the emergency use authorization for its COVID-19 antibody treatment. FDA gives emergency OK to Lillys antibody treatment for Covid-19 Nursing home residents who received the drug -- known as LY-CoV555 also called bamlanivimab --.
Washington US April 17 ANI. The US Food and Drug Administration FDA said on Friday that it has revoked it emergency authorisation of Eli Lillys COVID-19 monoclonal antibody treatment bamlanivimab when used on its own. Regulators for its bamlanivimab antibody treatment for.
Lilly scientists rapidly developed the antibody in just three months after AbCellera and the Vaccine Research Center at the National Institute of Allergy and Infectious. VANCOUVER British Columbia and INDIANAPOLIS March 12 2020 PRNewswire -- AbCellera and Eli Lilly and Company NYSE. LLY 160 said Friday it is seeking a revocation of the emergency use authorization granted by US.
Government has halted shipments of the coronavirus antibody treatment bamlanivimab which was developed by Eli Lilly. This investigational medicine referred to as LY-CoV555 is the first to emerge from the collaboration between Lilly and AbCellera to create antibody therapies for the prevention and treatment of COVID-19. The company is asking US.
The company based its decision on the availability of bamlanivimab and etesevimab together as well as the evolving variant COVID-19. A study in the New England Journal of Medicine NEJM this week shows that Eli Lillys COVID-19 convalescent plasmaderived virus-neutralizing monoclonal antibody treatment LY-CoV555 reduced viral loads lowered the severity of symptoms and had no serious adverse effects in outpatients.
 Eli Lilly S Covid 19 Treatment Drug Could Be Authorised For Use By September Chief Scientist
Eli Lilly S Covid 19 Treatment Drug Could Be Authorised For Use By September Chief Scientist
 New Covid 19 Treatments Are Here But Who Gets Them
New Covid 19 Treatments Are Here But Who Gets Them
Fda Oks Eli Lilly Covid 19 Drug But Supplies Will Be Limited Wbfo
 Eli Lilly S Covid 19 Antibody Begins Trials
Eli Lilly S Covid 19 Antibody Begins Trials
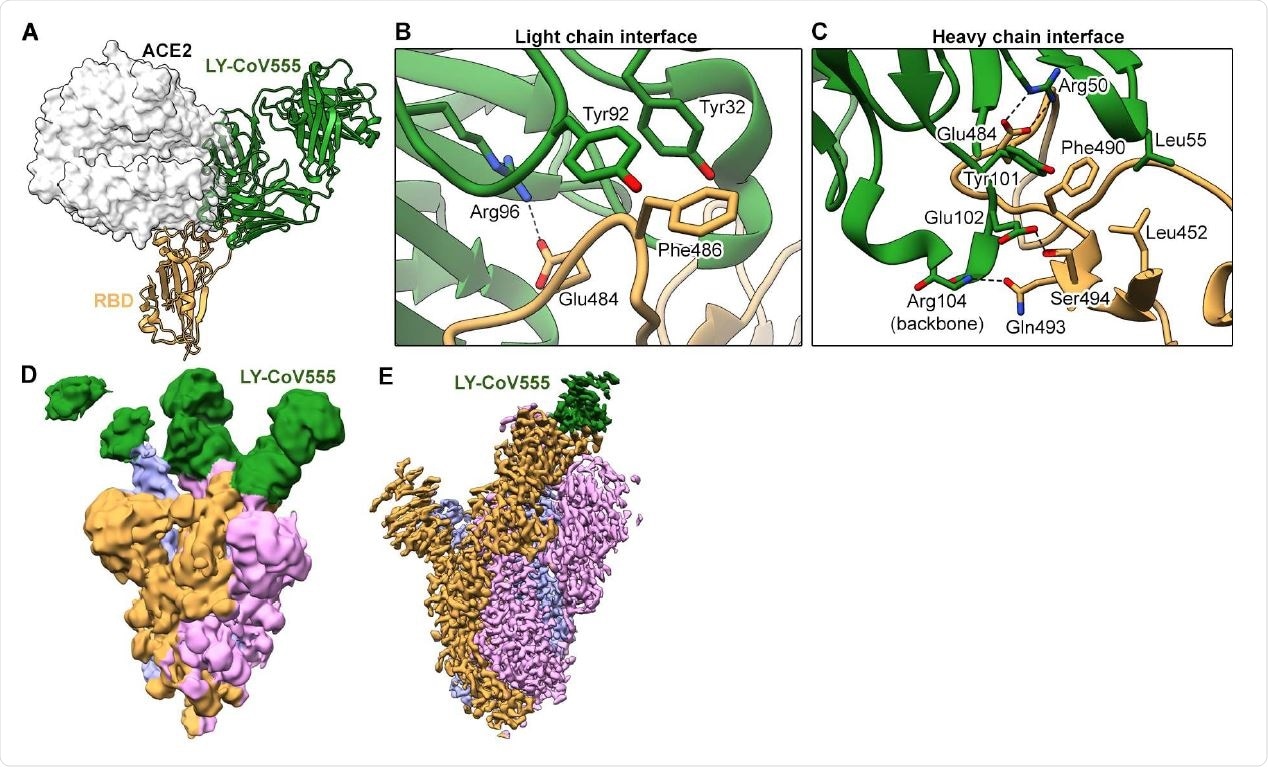 Eli Lilly S Ly Cov555 Antibody Shows Potential As Covid 19 Treatment
Eli Lilly S Ly Cov555 Antibody Shows Potential As Covid 19 Treatment
 Eli Lilly S Coronavirus Antibody Treatment Trial Halted
Eli Lilly S Coronavirus Antibody Treatment Trial Halted
 Covid 19 Monoclonal Antibody Treatment Available At Signaturecare Er
Covid 19 Monoclonal Antibody Treatment Available At Signaturecare Er
 Bamlanivimab Miracle Covid 19 Treatment Says Chicago Woman After Receiving Eli Lilly Antibody Infusion Abc7 Chicago
Bamlanivimab Miracle Covid 19 Treatment Says Chicago Woman After Receiving Eli Lilly Antibody Infusion Abc7 Chicago
 Lilly Begins World S First Study Of A Potential Covid 19 Antibody Treatment In Humans
Lilly Begins World S First Study Of A Potential Covid 19 Antibody Treatment In Humans
 Eli Lilly And Unitedhealth Group Partner On Covid 19 Antibody Treatment For High Risk Individuals Healthcare Finance News
Eli Lilly And Unitedhealth Group Partner On Covid 19 Antibody Treatment For High Risk Individuals Healthcare Finance News
 Eli Lilly S Coronavirus Antibody Treatment Trial Paused To Ensure The Safety Of Participants Health News Us News
Eli Lilly S Coronavirus Antibody Treatment Trial Paused To Ensure The Safety Of Participants Health News Us News
 Lilly Seeks Eua For Covid 19 Antibody Treatment 2020 10 07 Bioworld
Lilly Seeks Eua For Covid 19 Antibody Treatment 2020 10 07 Bioworld
 Eli Lilly Pauses Covid 19 Antibody Treatment Trial Over Safety Concern
Eli Lilly Pauses Covid 19 Antibody Treatment Trial Over Safety Concern
 Locating Antibody Treatments For Covid 19 Can Be A Treasure Hunt Shots Health News Npr
Locating Antibody Treatments For Covid 19 Can Be A Treasure Hunt Shots Health News Npr
Fda Authorizes Eli Lilly Antibody Drug To Treat The Coronavirus
 Coronavirus U S In 375 Million Deal With Eli Lilly For Antibody Drug
Coronavirus U S In 375 Million Deal With Eli Lilly For Antibody Drug
 Lilly Covid 19 Antibody Treatment Would Come With Hefty Infusion Costs Shots Health News Npr
Lilly Covid 19 Antibody Treatment Would Come With Hefty Infusion Costs Shots Health News Npr
 Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr
Antibody Treatments For Covid 19 Are Worth The Effort Doctors Say Shots Health News Npr

Post a Comment for "Coronavirus Antibody Treatment Eli Lilly"