Coronavirus Vaccine In Us Trial
The worlds biggest COVID-19 vaccine test got underway July 27 with the first of 30000 planned volunteers. I know the need is out there and I just wanted to be part of the solution if that was possible said Swaim.
 Q A When Will A Covid 19 Vaccine Be Widely Available For All Children
Q A When Will A Covid 19 Vaccine Be Widely Available For All Children
Funded by the National Institutes of Health NIHs National Institute of Allergy and Infectious Diseases NIAID the trial has dosed its first participant.

Coronavirus vaccine in us trial. Regarding a few of the current coronavirus vaccines in particular the objective of the first stage of Pfizer and BioNTechs Phase 12 trial is to assess the safety tolerability and. NIAID is conducting and supporting clinical trials evaluating therapies and vaccine candidates against severe acute respiratory syndrome coronavirus type 2 SARS-CoV-2 the virus that causes coronavirus disease 2019 COVID-19 as well as studies of people who have recovered from infection. The first human trial testing a potential vaccine to prevent COVID-19 began Monday US.
Read more trending news. A fourth Phase 3 clinical trial evaluating an investigational vaccine for coronavirus disease 2019 COVID-19 has begun enrolling adult volunteers. 85 rows Today the FDA issued the first emergency use authorization EUA for a vaccine for the.
Novavax COVID-19 vaccine Learn more about US. KWCH - Childrens Mercy in Kansas City is leading the regional efforts of a nationwide pediatric COVID-19 vaccine clinical trial by participating in the Pfizer-BioNTech global. 8 hours agoInovio Pharmaceuticals shares were chopped down by nearly a third on Friday after the US government said it will discontinue funding for a late-phase trial of the companys COVID-19 vaccine.
A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun. COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov external icon. 2 days agoKANSAS CITY Mo.
AstraZeneca US trial finds vaccine 79 effective at preventing symptomatic COVID-19 Medical staff show AstraZeneca coronavirus vaccine during preparations at. 1 day agoPresident Joe Biden gets a COVID-19 vaccine. The company is in the middle of a phase 3 trial in the US and Mexico involving 30000 people.
Oxford University Pool via AP The pharmaceutical giant Pfizer has begun testing a new coronavirus vaccine in the United States. The vaccine known as mRNA-1273 was co-developed by the Cambridge Massachusetts-based biotechnology company Moderna Inc and the National Institute of Allergy and Infectious Diseases NIAID part of the. A pharmacist gives Jennifer Haller the first shot March 16 2020 in the first-stage safety study clinical trial of a potential vaccine for COVID-19 at the Kaiser Permanente Washington Health Research Institute in Seattle.
The trial is designed to evaluate if the investigational Janssen COVID-19 vaccine JNJ-78436725 can prevent symptomatic COVID-19 after a single dose regimen. She signed up in January with Cincinnatis Sterling Research Lab. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States.
This page will be updated as. A fourth Phase 3 clinical trial evaluating an investigational vaccine for coronavirus disease 2019 COVID-19 has begun enrolling adult volunteers. The first US clinical trial of a Covid-19 vaccine candidate which is Modernas mRNA-1273 has started at Kaiser Permanente Washington Health Research Institute KPWHRI in Seattle.
By Michelle Ewing Cox Media Group National Content Desk The AstraZeneca-Oxford University coronavirus vaccine was 79 effective at preventing symptomatic COVID-19 in a Phase 3 clinical trial conducted in the United States Peru and Chile according to an interim analysis of the data. The trial is designed to evaluate if the investigational Janssen COVID-19 vaccine JNJ-78436725 can prevent symptomatic COVID-19 after a. Finding a safe and effective vaccine to prevent infection from the new.
Demetrius FreemanThe Washington Post. Johnson Johnson halted clinical trials of its Covid-19 vaccine after a participant fell ill the second time that a front-runner developer has paused testing in the race to create a viable. Julie Swaim of Camby is one of the participants.
 Covid 19 Vaccine Details New Variants When You Can Get Vaccinated Hidden Fees Cnet
Covid 19 Vaccine Details New Variants When You Can Get Vaccinated Hidden Fees Cnet
 Your Top Covid 19 Vaccine Questions Answered As Fda Gives The Green Light Shots Health News Npr
Your Top Covid 19 Vaccine Questions Answered As Fda Gives The Green Light Shots Health News Npr
 Astrazeneca Covid Vaccine 79 Effective In U S Trial
Astrazeneca Covid Vaccine 79 Effective In U S Trial
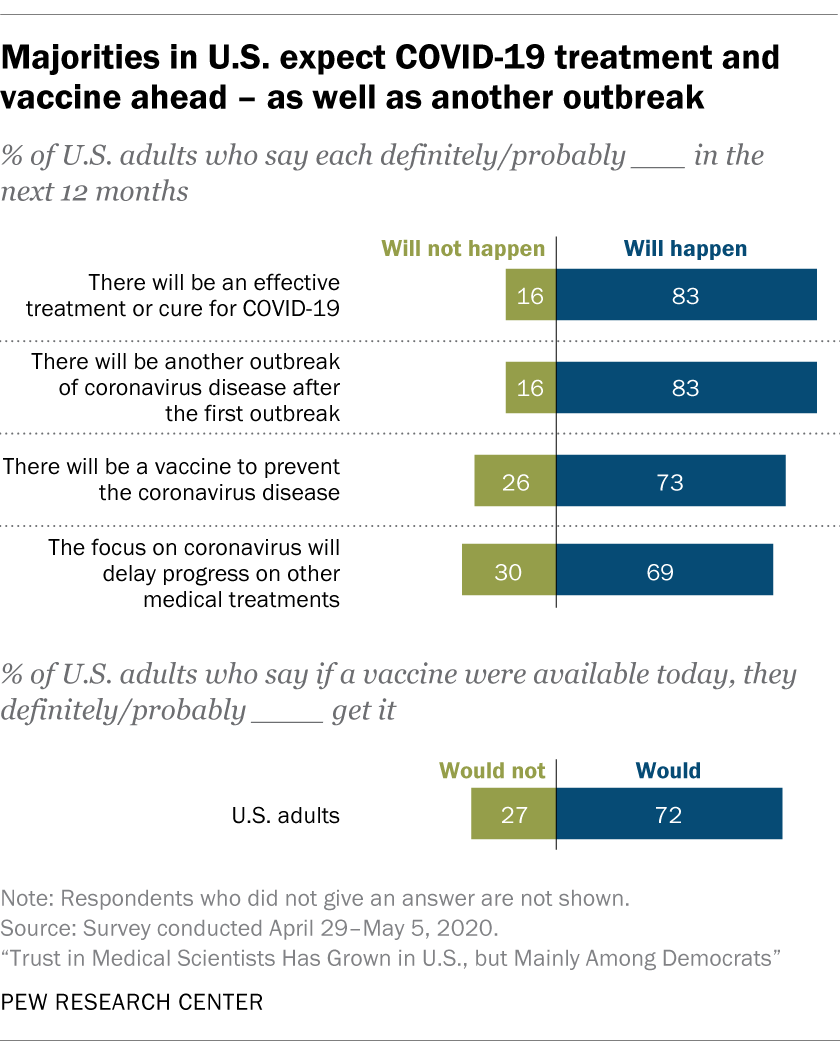 Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
 Astrazeneca S Covid 19 Vaccine Is 79 Percent Effective In U S Trial Science News
Astrazeneca S Covid 19 Vaccine Is 79 Percent Effective In U S Trial Science News
 China Begins Phase I Clinical Trial Of Covid 19 Vaccine
China Begins Phase I Clinical Trial Of Covid 19 Vaccine
 Us Coronavirus Black Community Not Well Represented In Covid 19 Vaccine Trials Abc11 Raleigh Durham
Us Coronavirus Black Community Not Well Represented In Covid 19 Vaccine Trials Abc11 Raleigh Durham
 Covid 19 Vaccine From Pfizer And Biontech Is Strongly Effective Data Show
Covid 19 Vaccine From Pfizer And Biontech Is Strongly Effective Data Show
 A Top Vaccine Expert Answers Important Questions About A Covid 19 Vaccine Covid 19 Johns Hopkins Bloomberg School Of Public Health
A Top Vaccine Expert Answers Important Questions About A Covid 19 Vaccine Covid 19 Johns Hopkins Bloomberg School Of Public Health
 Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
 When Will Americans Actually Get The Covid Vaccine Officials Offer Different Timelines
When Will Americans Actually Get The Covid Vaccine Officials Offer Different Timelines
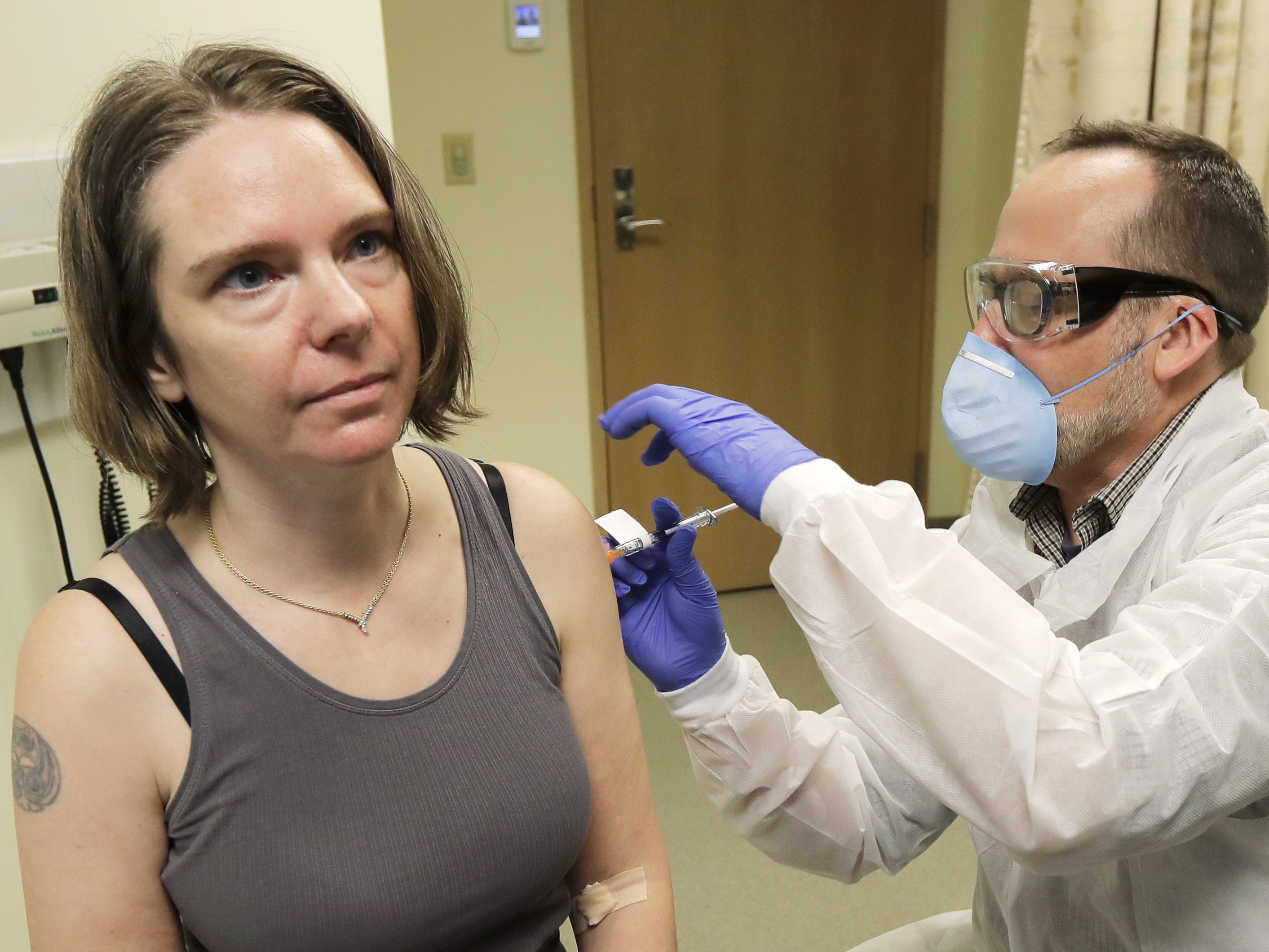 I Wanted To Do Something Says Mother Of 2 Who Is First To Test Coronavirus Vaccine Npr
I Wanted To Do Something Says Mother Of 2 Who Is First To Test Coronavirus Vaccine Npr
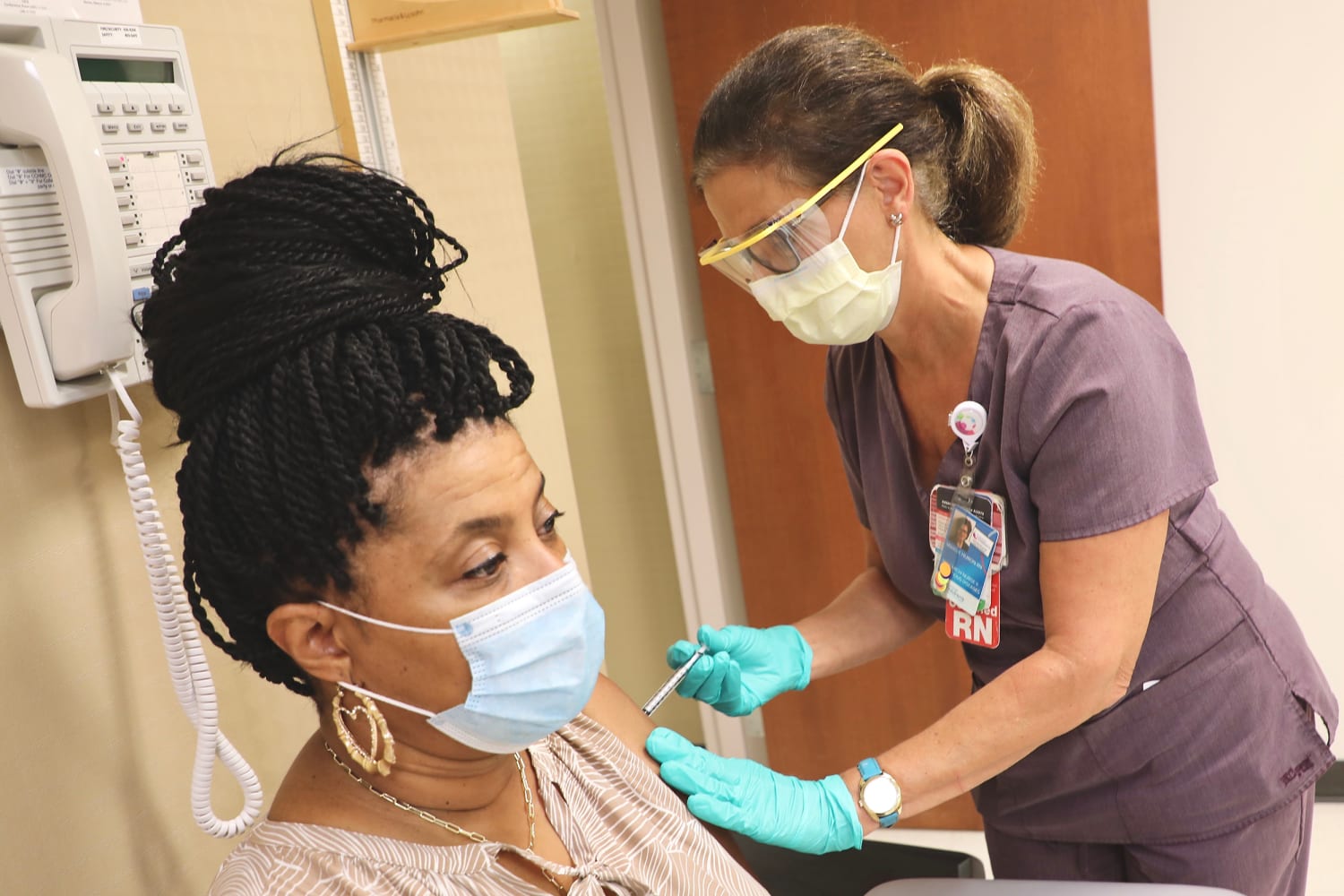 Pfizer S Covid 19 Vaccine Promising But Many Questions Remain
Pfizer S Covid 19 Vaccine Promising But Many Questions Remain
 Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
 Coronavirus Vaccine 90 Effective Say Pfizer And German Company Biontech News Dw 09 11 2020
Coronavirus Vaccine 90 Effective Say Pfizer And German Company Biontech News Dw 09 11 2020
 Covid New York First Covid Vaccination In Us Given To New York City Critical Care Nurse Abc7 New York
Covid New York First Covid Vaccination In Us Given To New York City Critical Care Nurse Abc7 New York
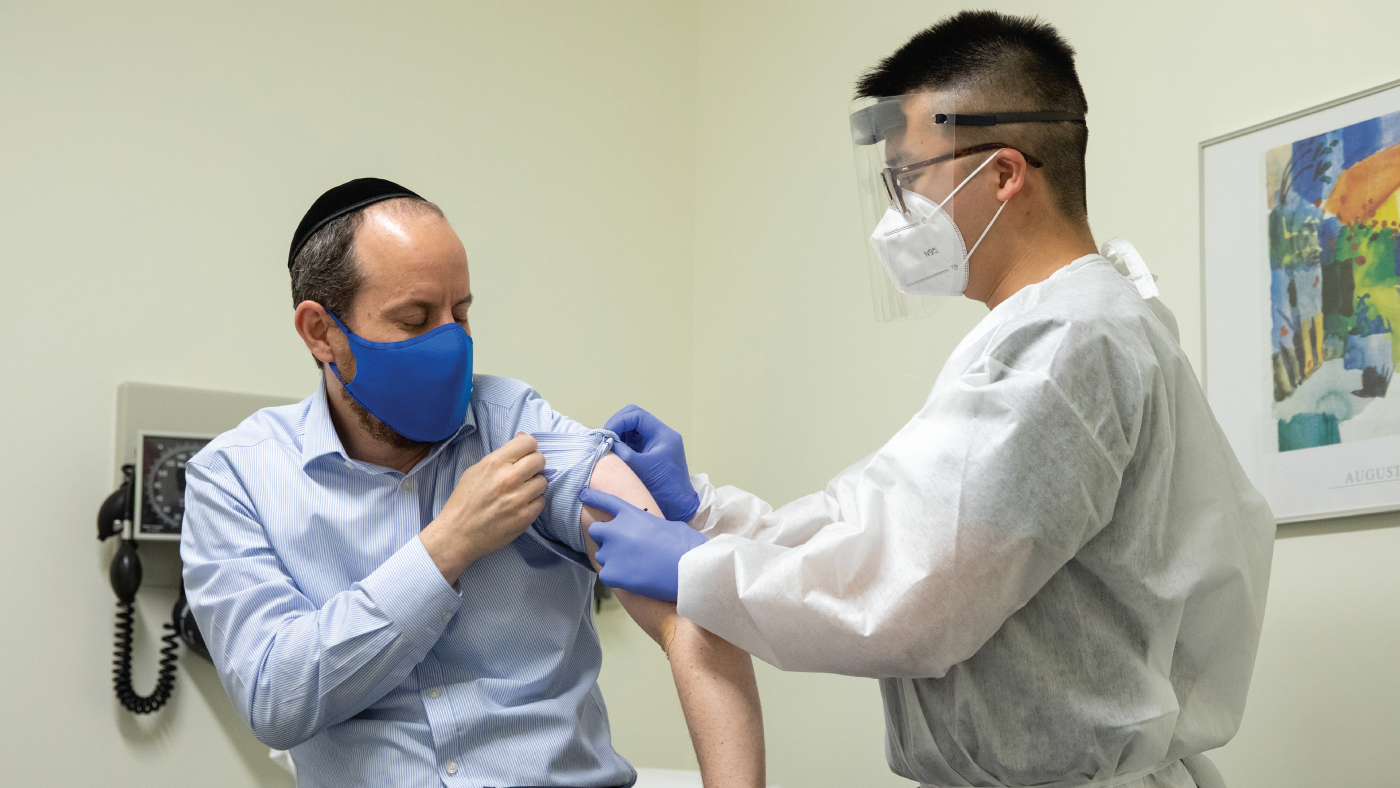 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
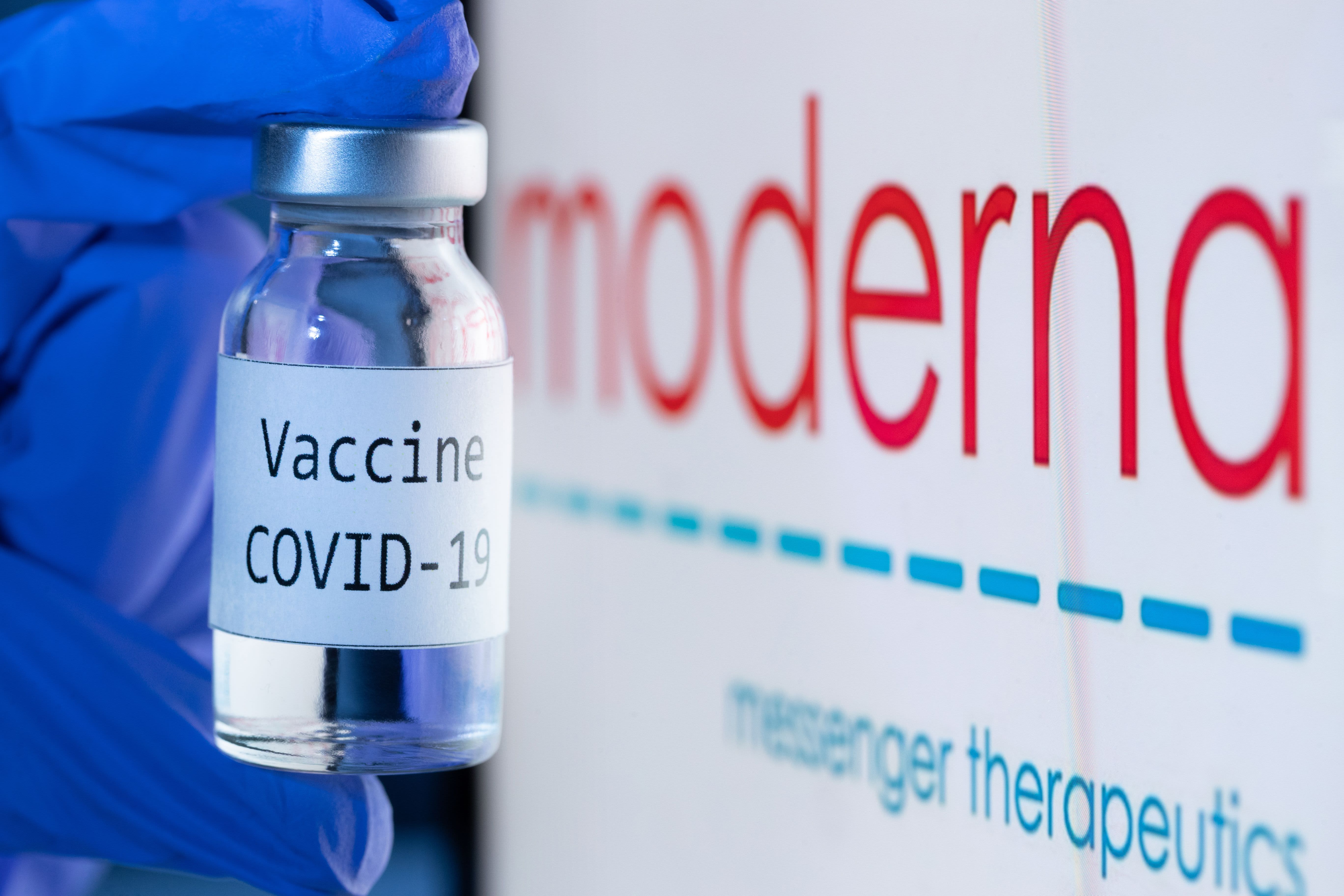 Fda Staff Recommends Watching For Bell S Palsy In Moderna And Pfizer Vaccine Recipients
Fda Staff Recommends Watching For Bell S Palsy In Moderna And Pfizer Vaccine Recipients
 First Human Trial Results Raise Hopes For Coronavirus Vaccine Vaccines And Immunisation The Guardian
First Human Trial Results Raise Hopes For Coronavirus Vaccine Vaccines And Immunisation The Guardian
Post a Comment for "Coronavirus Vaccine In Us Trial"