Covid 19 Antibody Test Kit Home Use
The Everlywell COVID-19 Test Home Collection Kit allows qualifying individuals to collect a sample and send it to a certified lab to receive digital results within 24-48 hours. 8 hours agoMegna Health announced today that its Rapid COVID-19 IgMIgG Test previously authorized for use under the US.
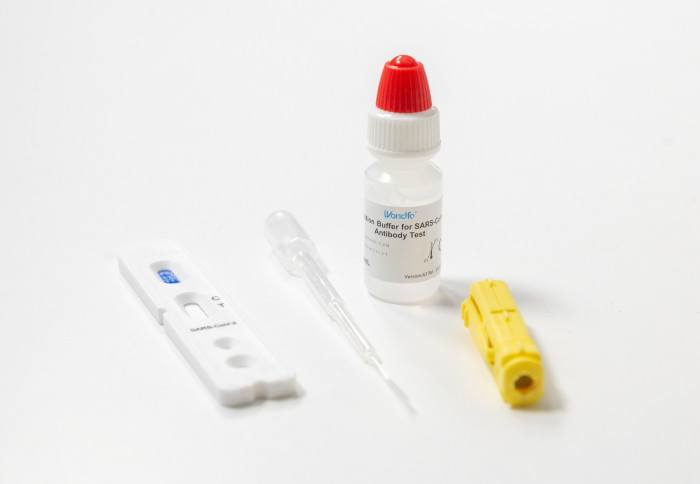 Self Test Kits Can Monitor The Spread Of Covid 19 In Communities Imperial News Imperial College London
Self Test Kits Can Monitor The Spread Of Covid 19 In Communities Imperial News Imperial College London
9 rows Antibody tests are not the same as at-home coronavirus test kits like the.
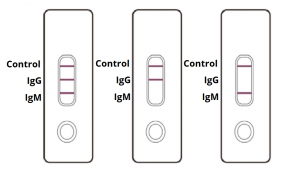
Covid 19 antibody test kit home use. MailOnline Be sure to thoroughly wash your hands and dry them properly before you do anything else. 10 hours agoClinical Enterprise Incs EmpowerDX At-Home COVID-19 PCR Test Kit issued on October 15 2020. Ambient room temperature draw kits are available for this test.
Antibody testing sometimes referred to as serology testing cannot be used to diagnose an individual with COVID-19. Everlywell offers this test and collection kit with an FDA Emergency Use Authorization EUA. Food and Drug Administration FDA Emergency Use Authorization is now available for.
Food and Drug Administration FDA Emergency Use. A positive result may indicate whether an individual has been exposed to and mounted an immune response to the virus. The test kit is made by LabCorp and is known as the Pixel by LabCorp COVID-19 Test home collection kit using COVID-19 RT-PCR technology.
It is important to remember that COVID-19 antibody testing should not be used to determine or validate the effectiveness of a COVID-19 vaccine. A guide on how to use a coronavirus antibody testing kit from home Picture. Food and Drug Administration FDA.
The test which spots the presence of virus-fighting antibodies rather than a coronavirus infection can be adapted to work on blood from a finger prickmaking it quick and easy to use. Special Instructions Forms. Use promo code GOODRX for 15 off.
DNA Genotek Incs ORAcollectRNA OR-100 and ORAcollectRNA ORE-100 saliva collection devices issued October 28 2020. If you suspect you have COVID-19 follow up with your healthcare provider about getting a PCR test. Your sample is returned to LabCorp to determine the result.
This test cannot tell you if you have an active infection. On April 20th the US Food and Drug Administration FDA authorized the first at-home COVID-19 test collection kit so you can test yourself at home. Rated 47 on Google Shopping.
The kits will use Celltrions COVID-19 antibody-antiviral technology to enhance their detection sensitivity. PRWEB April 23 2021 Megna Health announced today that its Rapid COVID-19 IgMIgG Test previously authorized for use under the US. Your MinuteClinic practitioner will perform the antibody test and review your results with you.
COVID-19 Antibody Spike IgG Titer COVID-19 Antibody Nucleocapsid IgG Titer. Binx health Incs binx health At-Home Nasal Swab COVID-19 Sample Collection Kit issued October 20 2020. Use an alcohol swab.
12 hours agoEXTON Pa. The FDA first authorized the test for use by trained personnel in August touting it at the time as the first Covid-19 test that costs about 5 and delivers results in minutes on a testing card. The FDA granted emergency use authorization to the first at-home over-the-counter antigen test for COVID-19 that can be used in individuals ages 2 and up including those not showing symptoms.
This test should not be used to determine the level of immunity you have. COVID-19 SARS-CoV-2 Antibody Test Serological antibody tests for COVID-19 are used to detect the presence of antibodies against the SARS-CoV-2 virus. 11 hours agoEXTON Pa April 23 2021 PRNewswire-PRWeb -- Megna Health announced today that its Rapid COVID-19 IgMIgG Test previously authorized for use under the US.
An antibody test will show whether or not you have developed antibodies to COVID-19 after exposure or vaccination. Instead physicians use antibody tests to determine if patients have been. The authorization of the first prescription-use home collection antibody test will play an important role in helping healthcare professionals identify individuals who have developed an adaptive.
Celltrion requested EUAs for both test kits in July 2020 and was also planning to launch second-generation rapid antibody and antigen diagnostic kits also co-developed with Humasis. If you would like to receive COVID-19 Antibody draw kits that include free shipping for hospital facilities please go to this page. Up to 4 cash back An antibody test can detect antibodies that developed as a result of exposure to COVID-19.
 Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
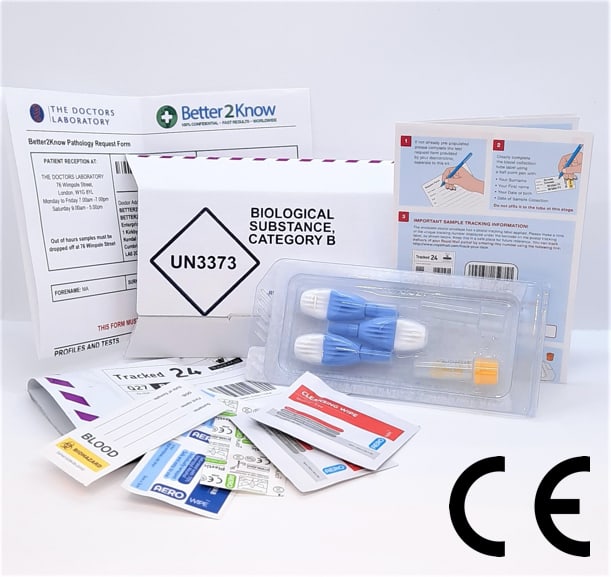 Covid 19 Quantitative Finger Prick Antibody Test Ce Marked Better2know
Covid 19 Quantitative Finger Prick Antibody Test Ce Marked Better2know
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
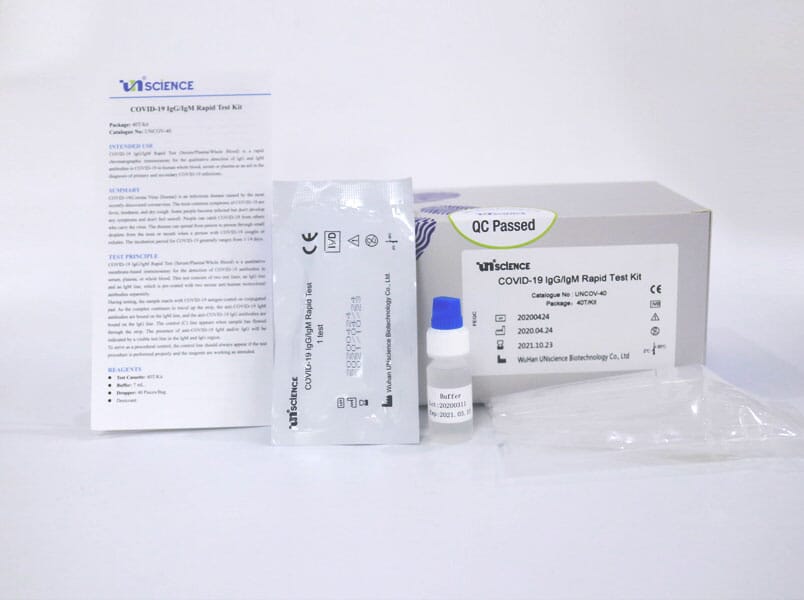 Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
Covid 19 Rapid Test Kit Igg Igm Colloidal Gold A122152
 Understanding Antibody Testing For Covid 19 The Journal Of Arthroplasty
Understanding Antibody Testing For Covid 19 The Journal Of Arthroplasty
 Abbott S Binaxnow Covid 19 Rapid Test Authorized For Home Use 2020 12 17 Bioworld
Abbott S Binaxnow Covid 19 Rapid Test Authorized For Home Use 2020 12 17 Bioworld
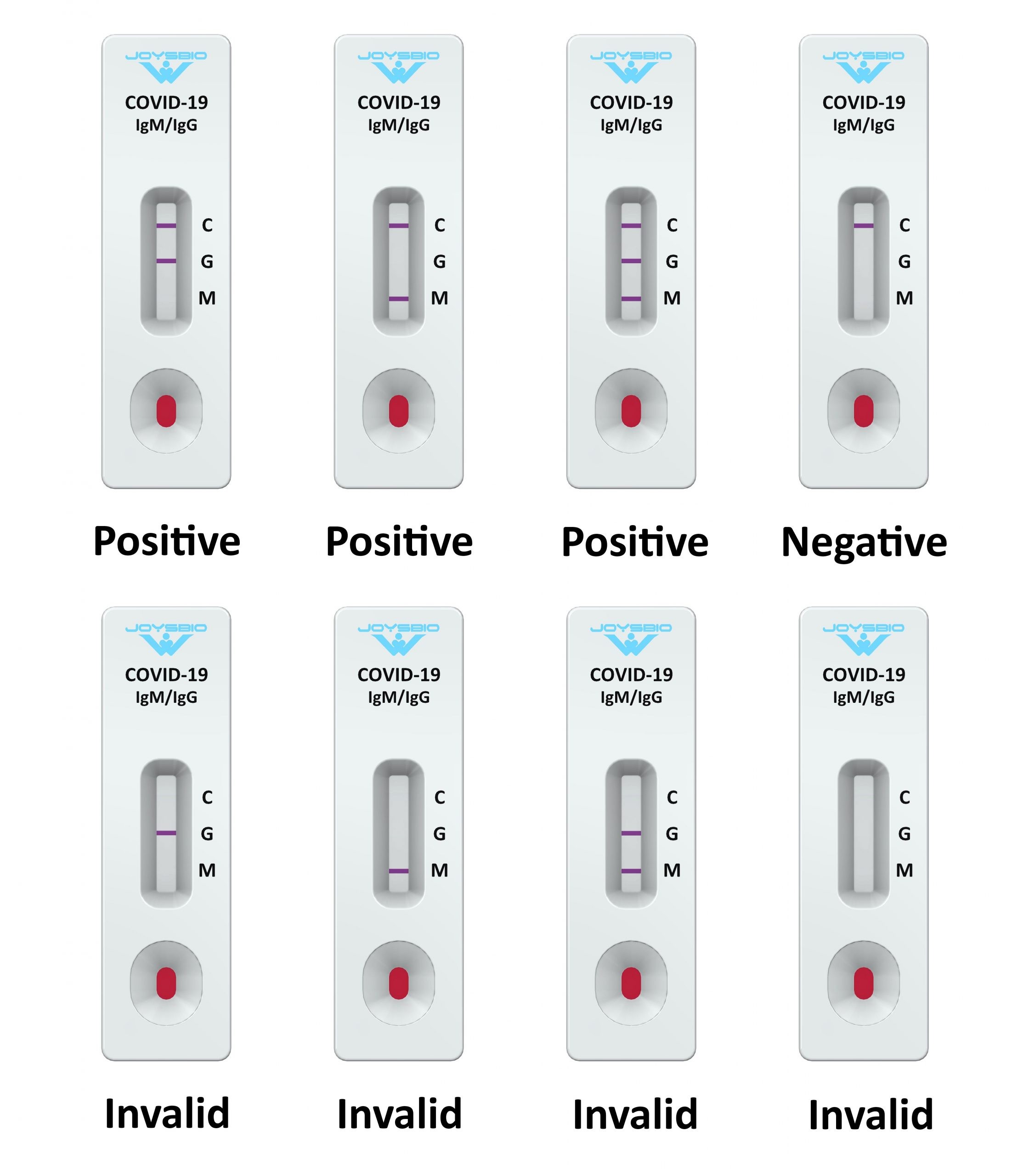 Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test
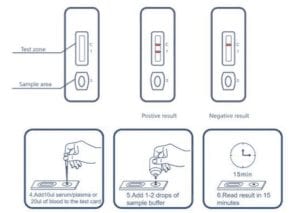 Coronavirus Covid 19 Antibody Test Glenbio
Coronavirus Covid 19 Antibody Test Glenbio
 Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igg Igm Rapid Test
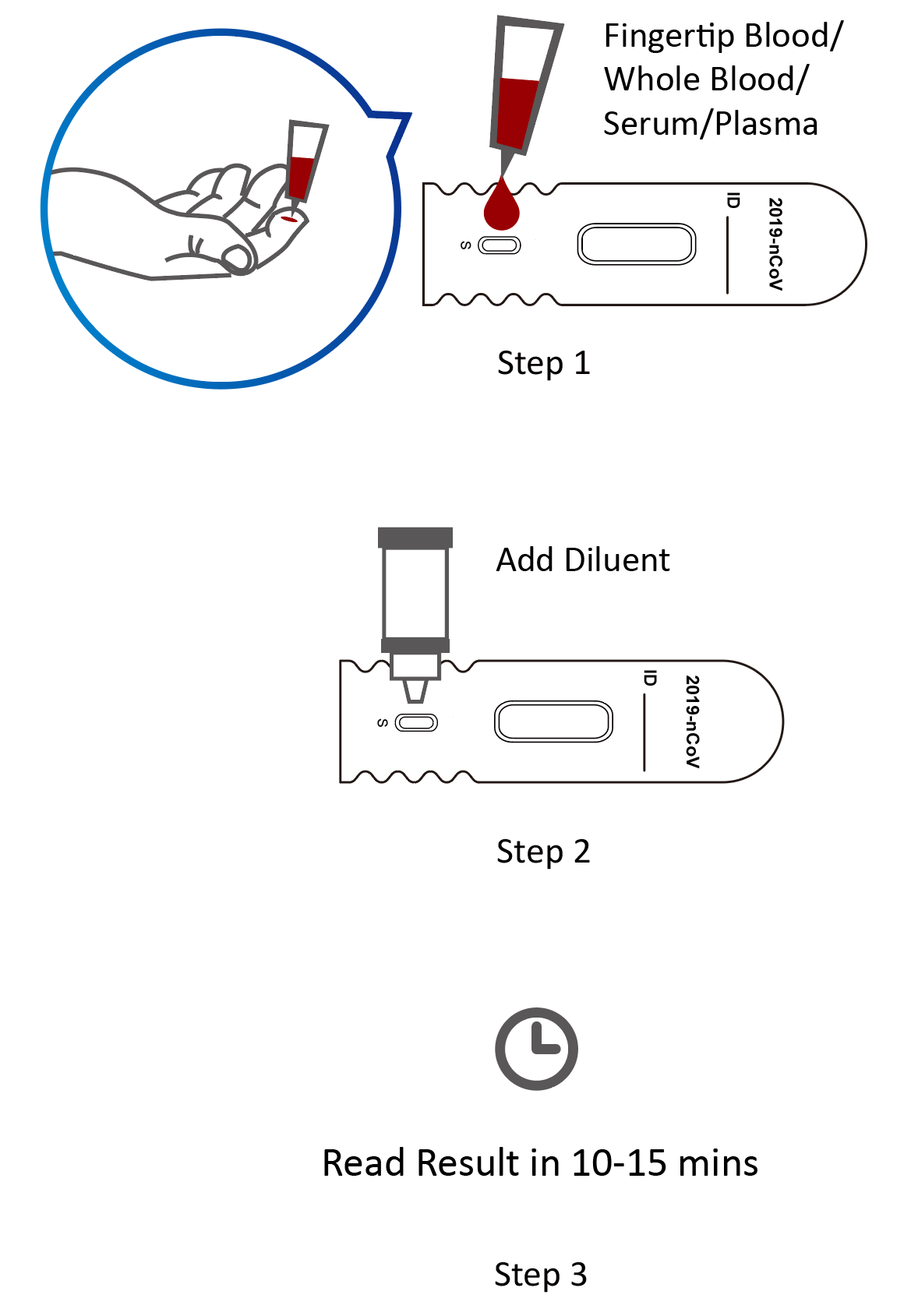 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
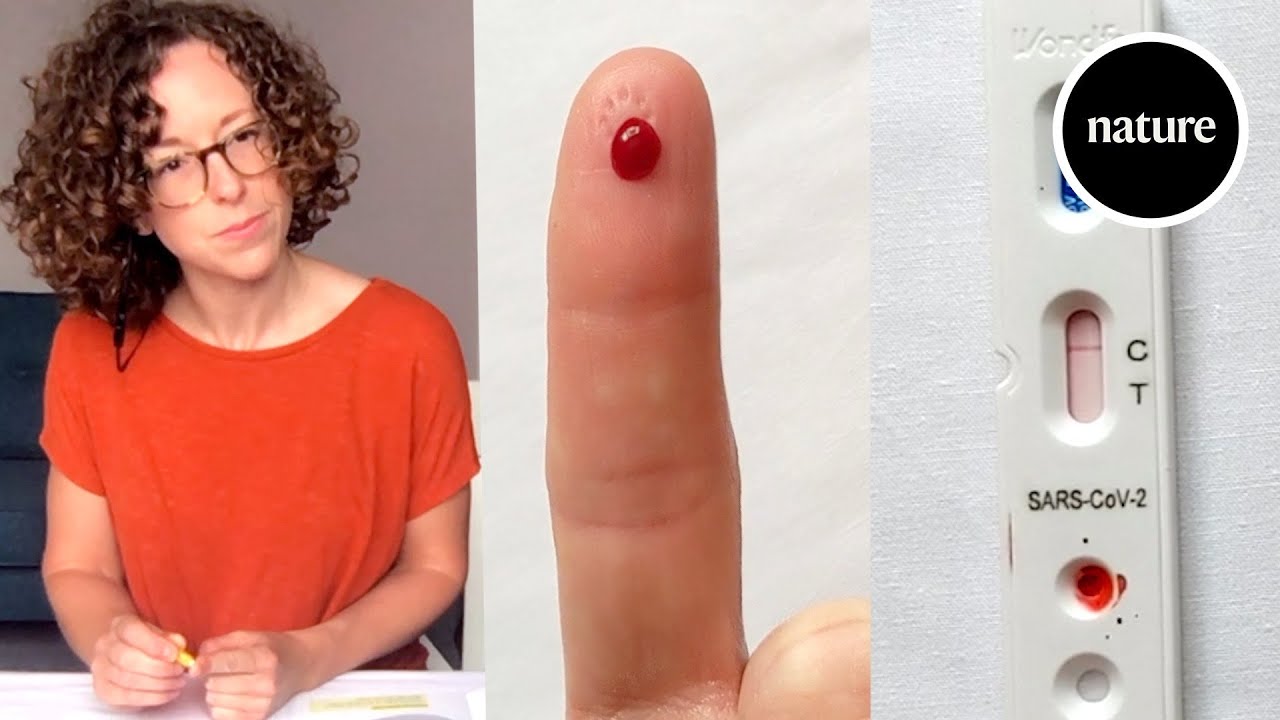 We Test A Home Antibody Kit For Tracking Covid 19 Transmission Youtube
We Test A Home Antibody Kit For Tracking Covid 19 Transmission Youtube
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
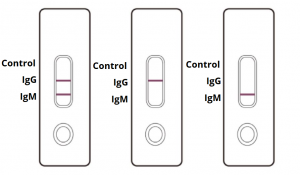 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 Sars Cov 2 Rapid Antibody Test
Sars Cov 2 Rapid Antibody Test
 The Potential And Limits Of Antibody Testing Covid 19 Johns Hopkins Bloomberg School Of Public Health
The Potential And Limits Of Antibody Testing Covid 19 Johns Hopkins Bloomberg School Of Public Health
 Antibody Testing Results Covid 19 Infections Exceed Confirmed Cases
Antibody Testing Results Covid 19 Infections Exceed Confirmed Cases
 Covid 19 Igg Igm Rapid Test Ce Ctk Biotech
Covid 19 Igg Igm Rapid Test Ce Ctk Biotech
 Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
 How Do Coronavirus Antibody Tests Work
How Do Coronavirus Antibody Tests Work
Post a Comment for "Covid 19 Antibody Test Kit Home Use"