Will There Be A Covid 19 Vaccine In 2020
The EUA means that there will be a. On December 18 2020 the US.
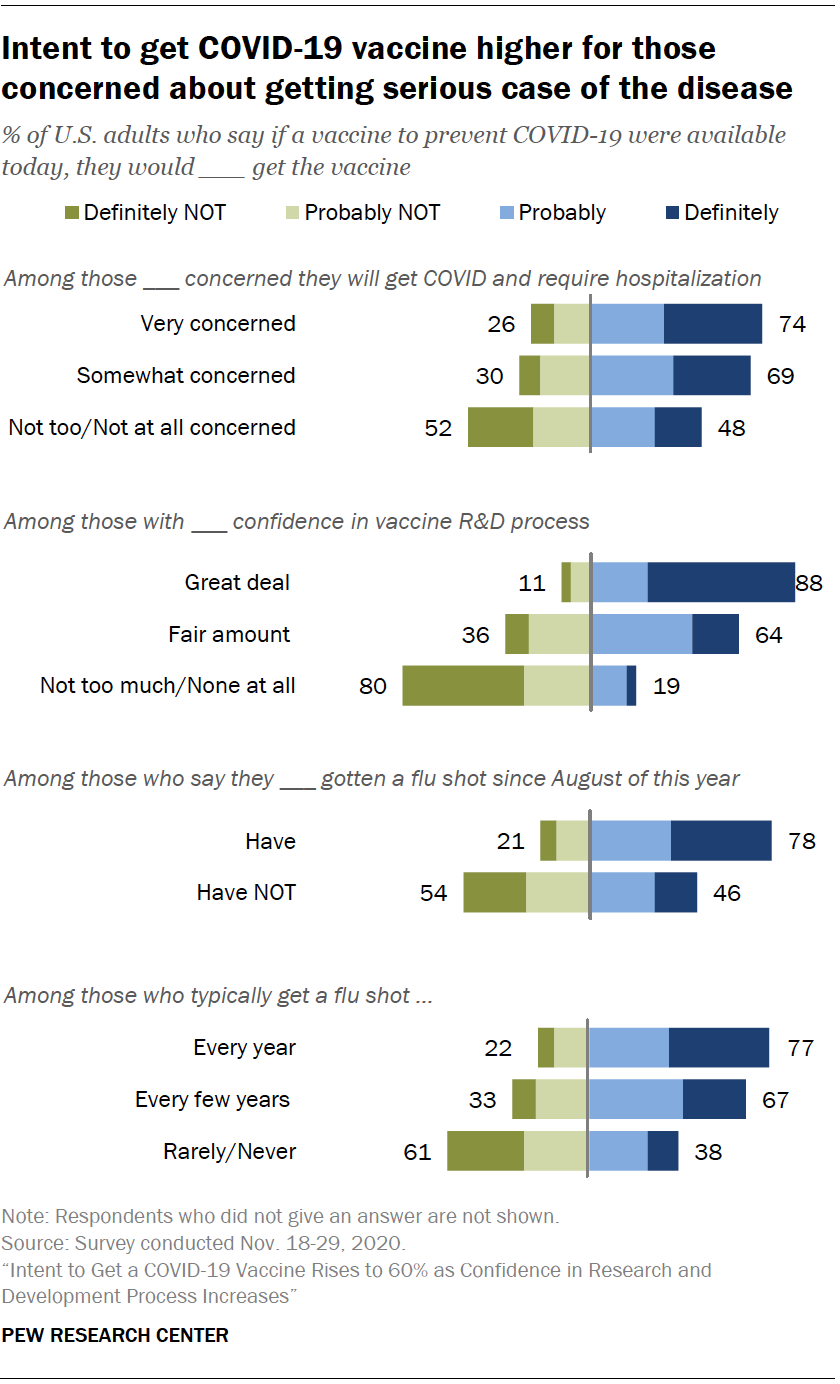 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
May 4 2020 - Today in the world of coronavirus news - People will likely need to take a coronavirus vaccine annually to protect themselves similar to the annual flu vaccine.
Will there be a covid 19 vaccine in 2020. 28 2020 655 PM. Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of coronavirus disease 2019 COVID-19 caused by. Vaccine or no vaccine theres no returning to the pre-COVID-19 world biosecurity expert says.
The written comment deadline was Wednesday October 21 2020. On October 14 2020 IHS initiated tribal consultation PDF and urban confer PDF on the IHS COVID-19 Pandemic Vaccination Draft Plan. Since we shared our perspectives on COVID-19-vaccine development in July 2020 the pandemic has grown in proportion across most of Europe and North America with more than a million new cases every two days and more than 10000 deaths per day.
Just over a month later on Dec. The COVAX AMC is critical to ensuring equitable access to COVID-19 vaccines regardless of income level and requires an urgent investment of US 2 billion from sovereign donors philanthropies and the private sector by the end of 2020. Crossing state lines to get a vaccine where there.
Fifty-eight percent of American adults said in a Gallup poll released in November that they would get a COVID-19 vaccine. Pfizer Moderna and how many vaccine doses are coming in 2020 Two vaccine candidates that claim to be about 95 effective against coronavirus will. IHS Consultation and Confer.
The most common reason cited for not wanting to get a vaccine was the. 1 day agoA subcommittee of vaccine advisers to US Centers for Disease Control and Prevention will recommend Friday that the Johnson Johnson Covid-19 vaccine continue to. Could release a COVID-19 vaccine before the electionand why that scares some.
Initial doses are limited but vaccinations have already started nationwide and additional vaccines could be authorized soon. About 1312 million people in the United States have received at least one dose of a Covid-19 vaccine. By Jon Cohen Aug.
The work on the. Prior to the COVID19 pandemic there was an established body of knowledge about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome. 85 rows FDA Commissioner Stephen Hahn and CBER Director Peter Marks discuss the EUA issued for the Moderna COVID-19 Vaccine December 18 2020.
COVAX Advance Market Commitment mechanism explained. A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus causing coronavirus disease 2019 COVID19. Population under an.
Heres how the US. The first COVID-19 vaccines will be released to the US. Less than a year into the coronavirus pandemic the US.
Because COVID-19 vaccines clinical trials only started in the summer of 2020 its not yet clear if these vaccines will have long-term side effects. Has authorized the first vaccine to help combat COVID-19 the devastating illness that has so far sickened millions and killed more than 300000 Americans. 11 the Food and Drug Administration granted it the first emergency use authorization ever given by the United States to a coronavirus vaccine.
On November 18 2020 IHS issued the IHS COVID-19 Pandemic Vaccine Plan November 2020 PDF. DRAFT landscape of COVID-19 candidate vaccines 2 December 2020. By Lachlan Gilbert University of New South Wales.
Food and Drug Administration authorizing the Pfizer COVID-19 vaccine the shot will start rolling out within days a long-awaited milestone in the coronavirus crisis for some and a. In this update we track the progress of COVID-19 vaccines and therapeutics as new clinical data and virus variants emerge.
 A Guide To Who Can Safely Get The Pfizer Biontech Covid 19 Vaccine
A Guide To Who Can Safely Get The Pfizer Biontech Covid 19 Vaccine
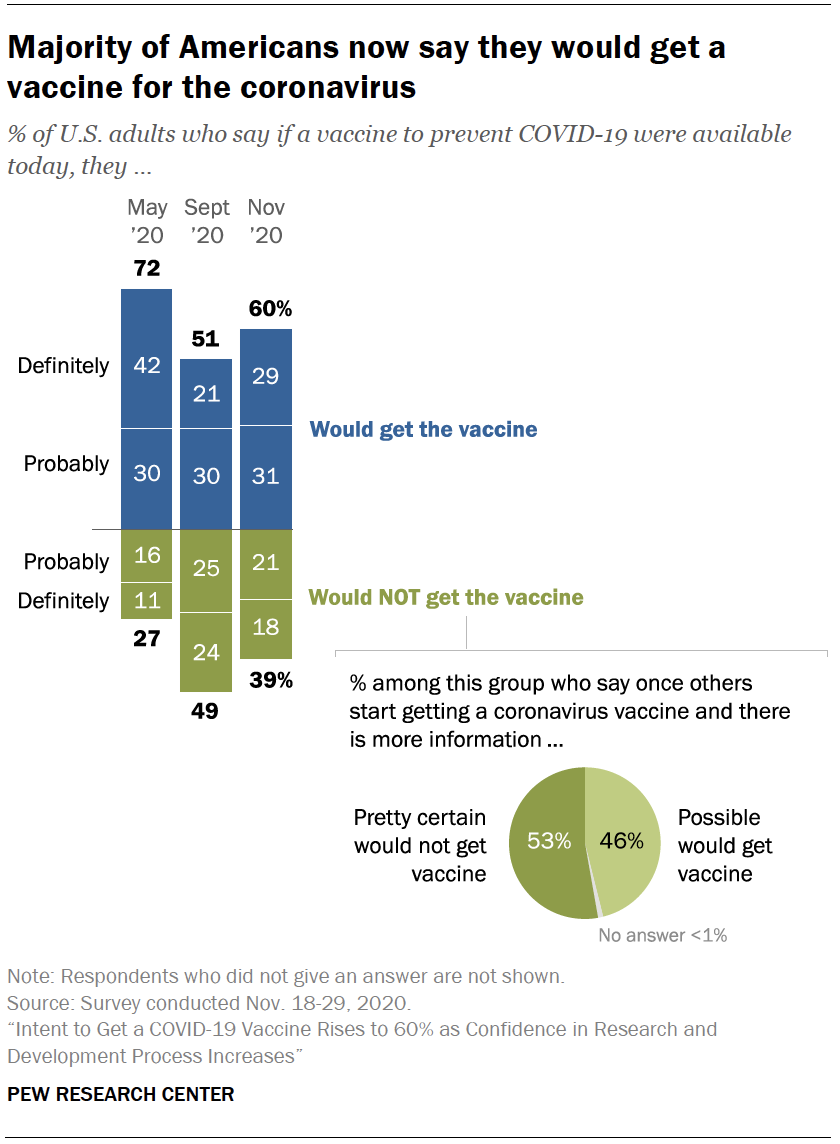
 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
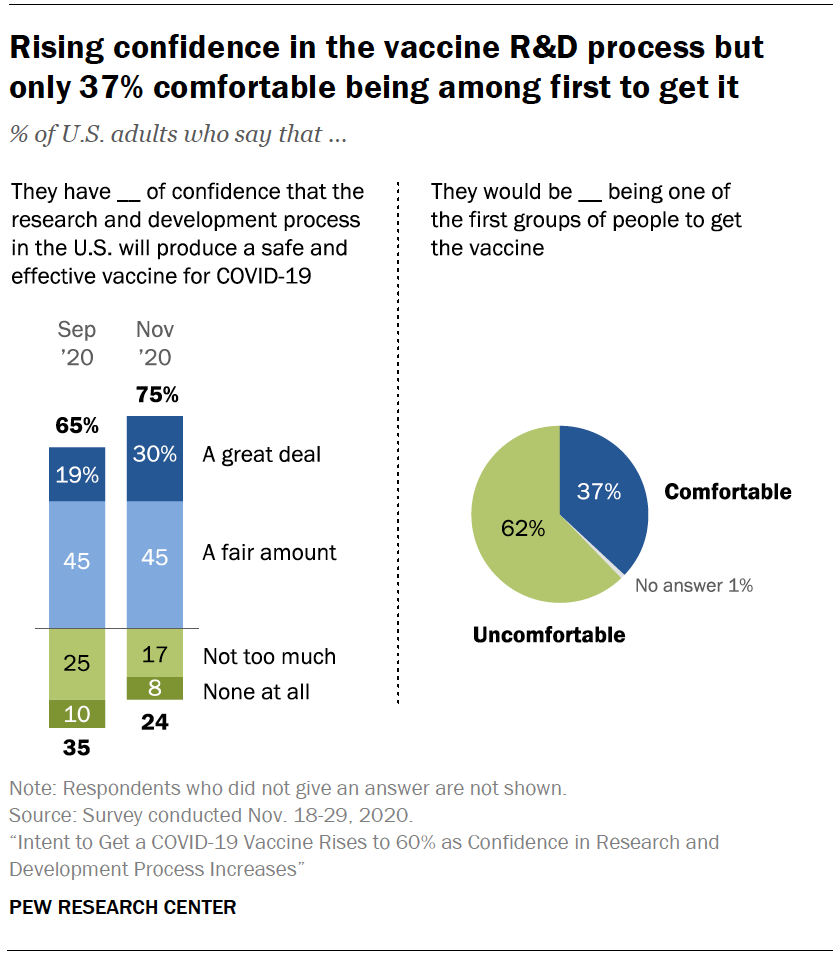
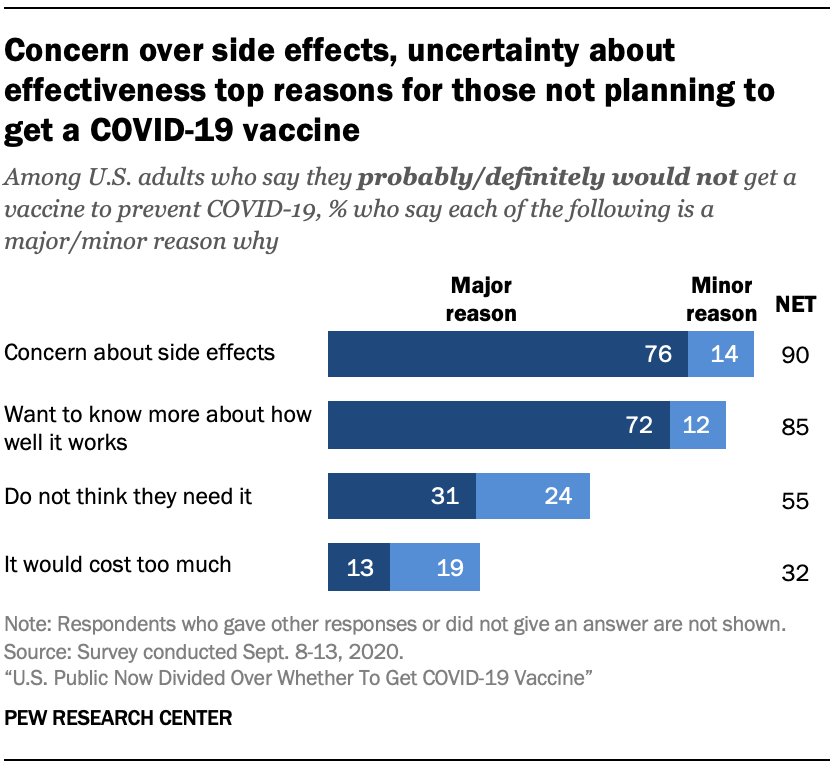 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
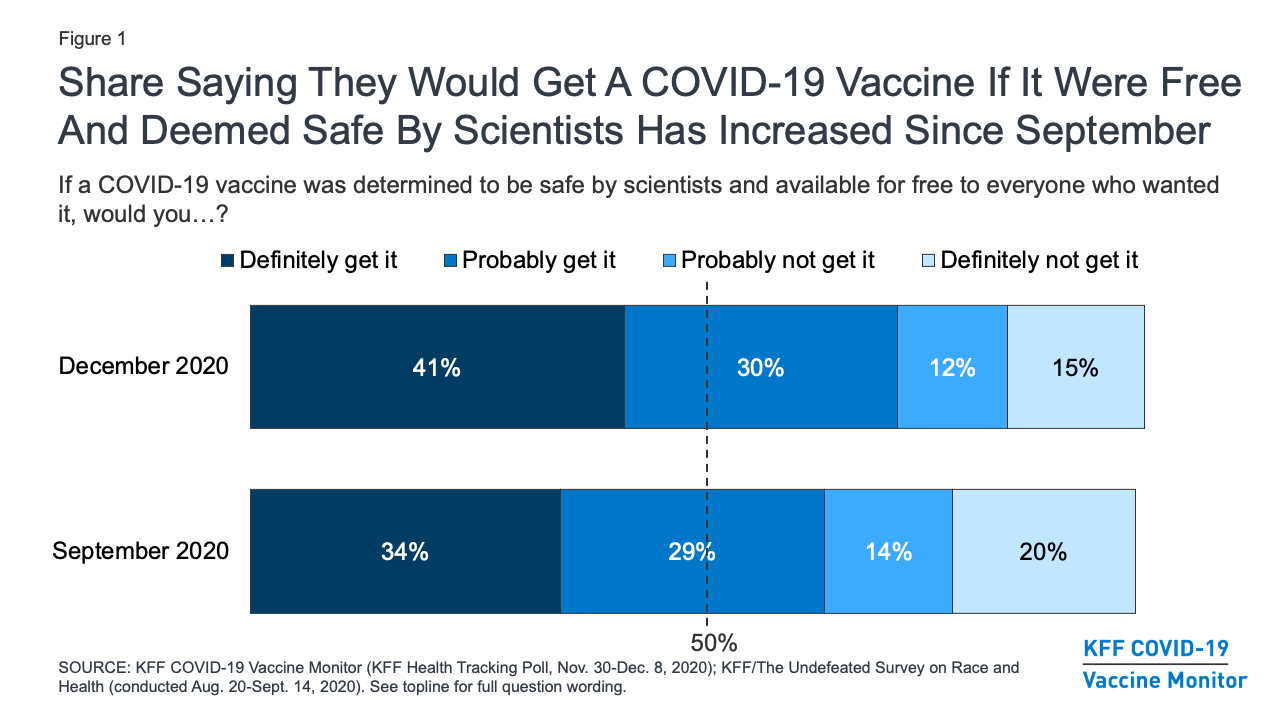 Kff Covid 19 Vaccine Monitor December 2020 Kff
Kff Covid 19 Vaccine Monitor December 2020 Kff
 Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 21 04 2021
Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 21 04 2021
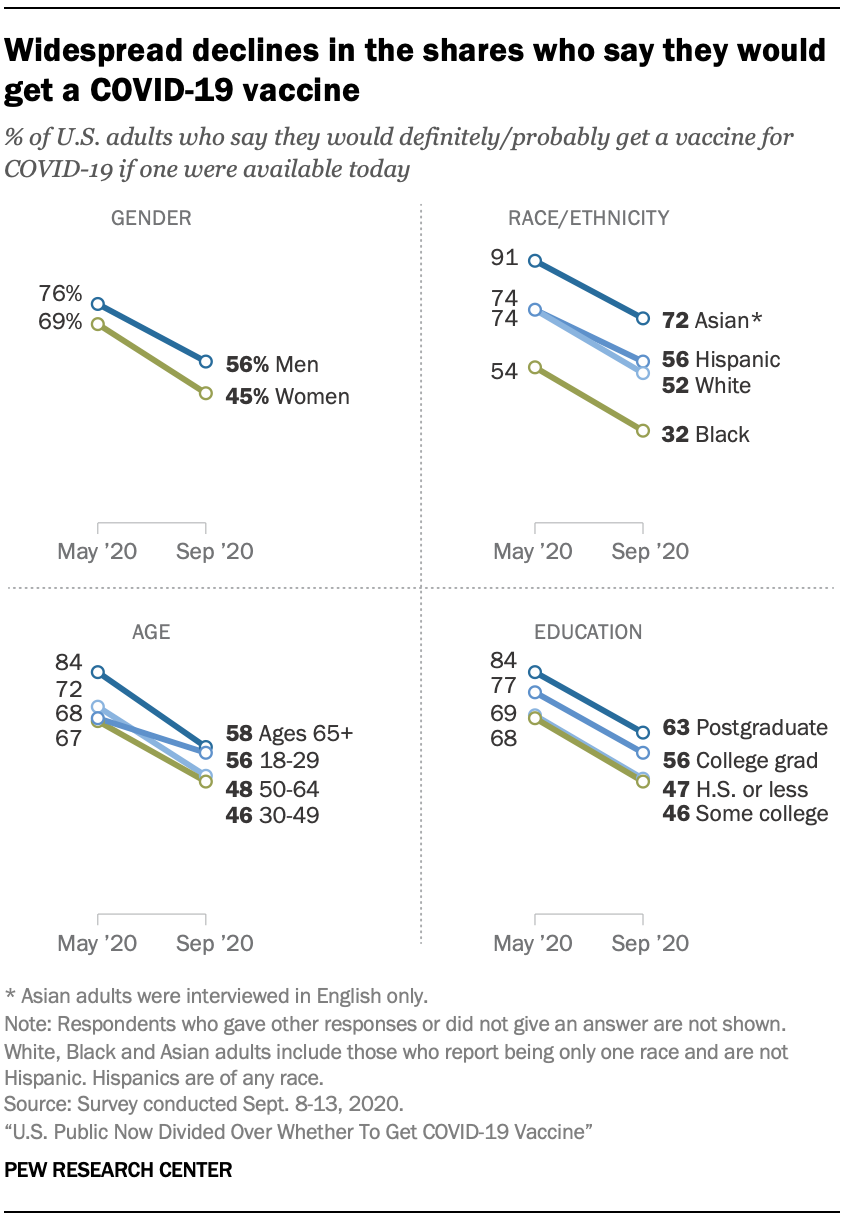 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
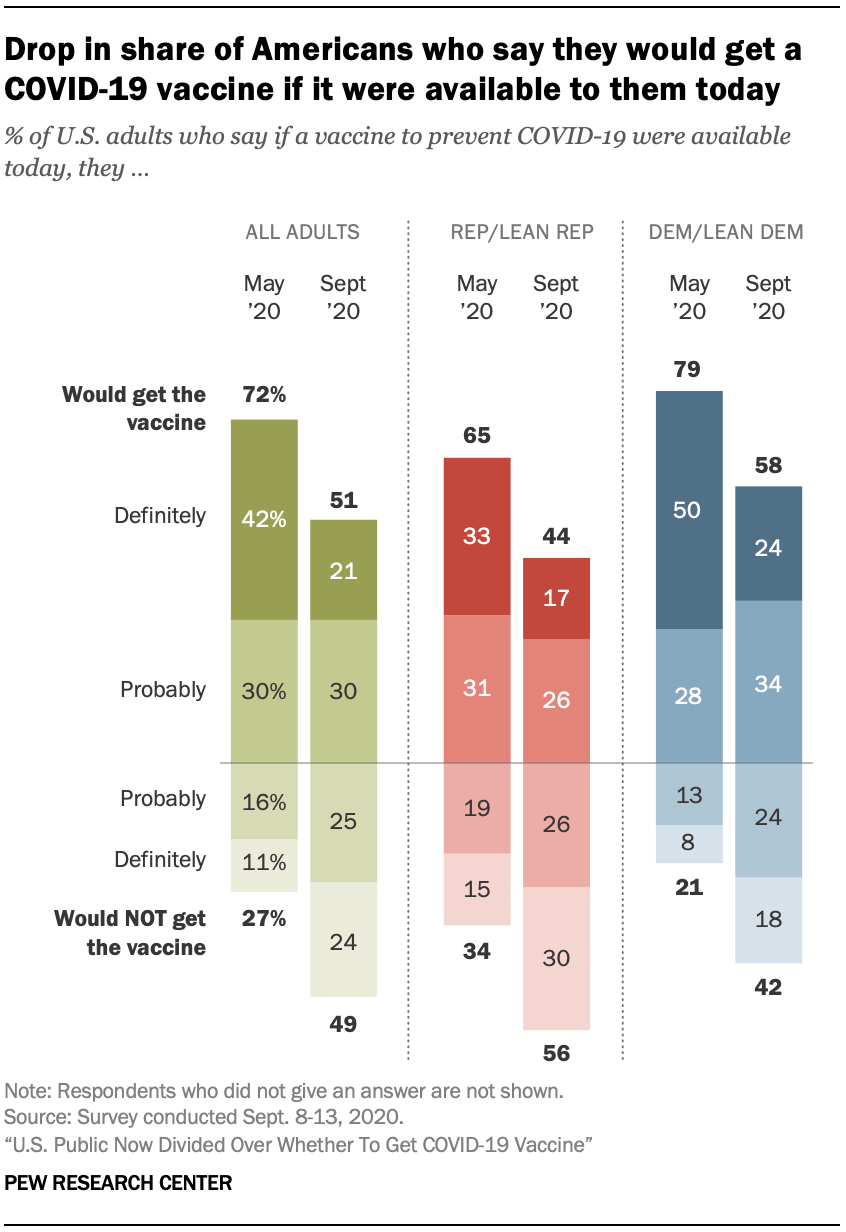 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
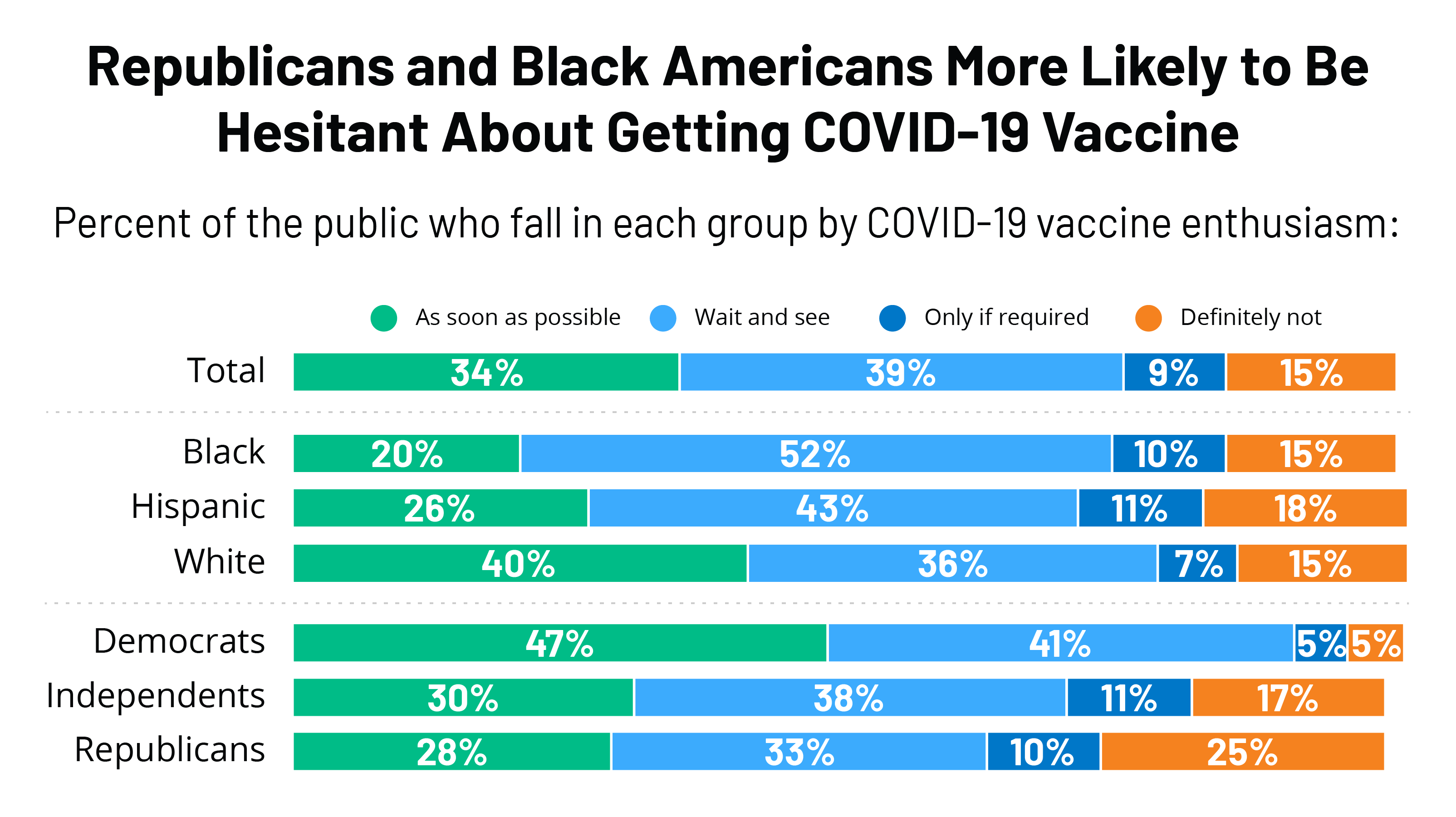 Kff Covid 19 Vaccine Monitor December 2020 Kff
Kff Covid 19 Vaccine Monitor December 2020 Kff
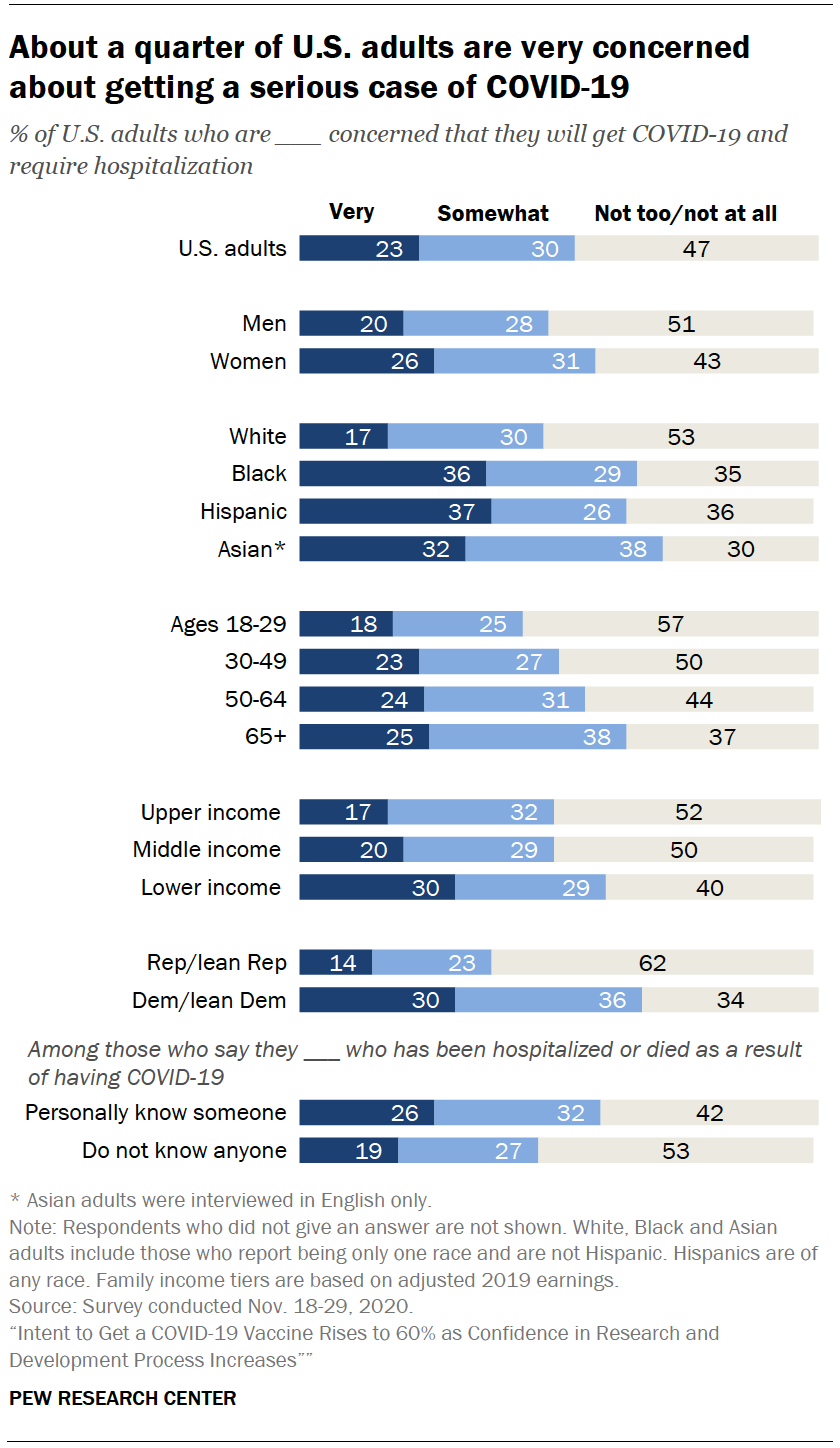 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
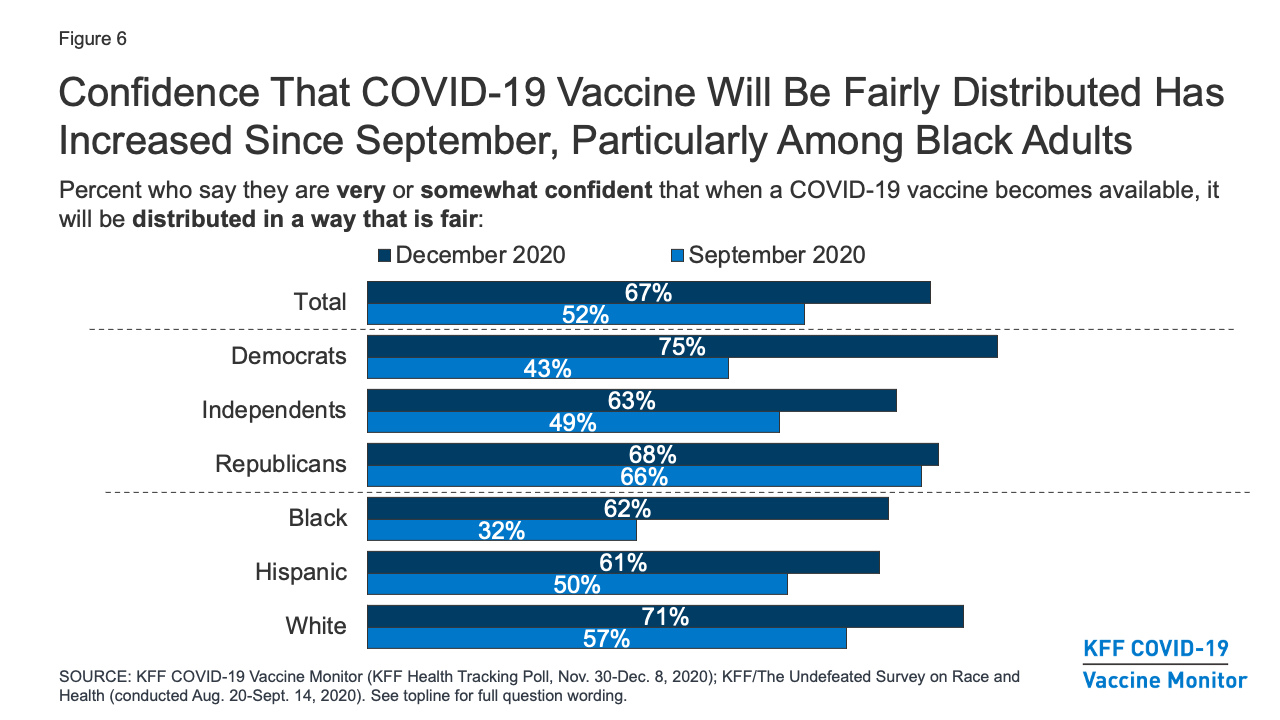 Kff Covid 19 Vaccine Monitor December 2020 Kff
Kff Covid 19 Vaccine Monitor December 2020 Kff
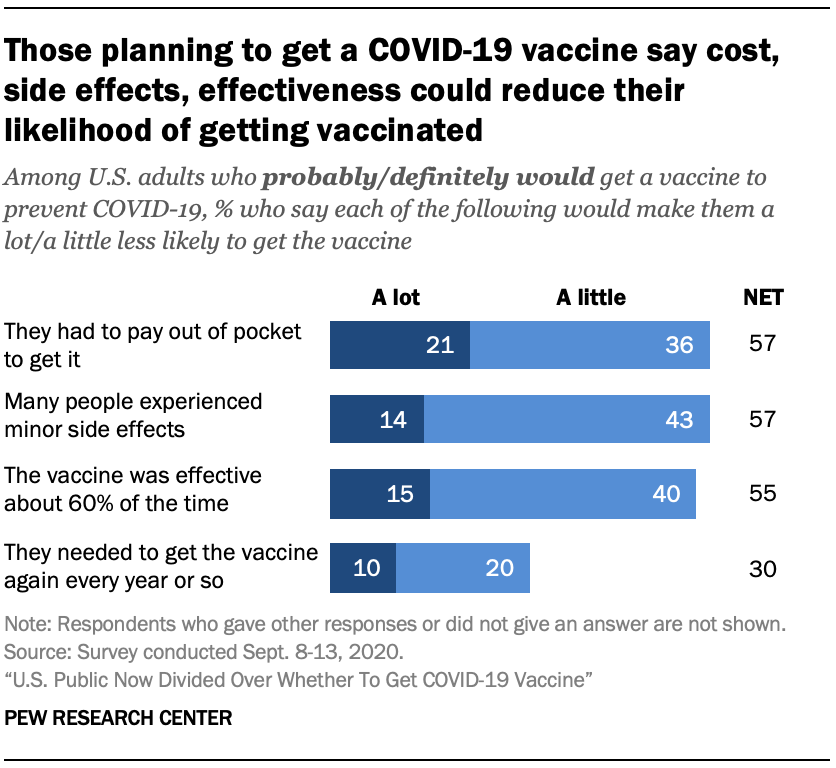 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
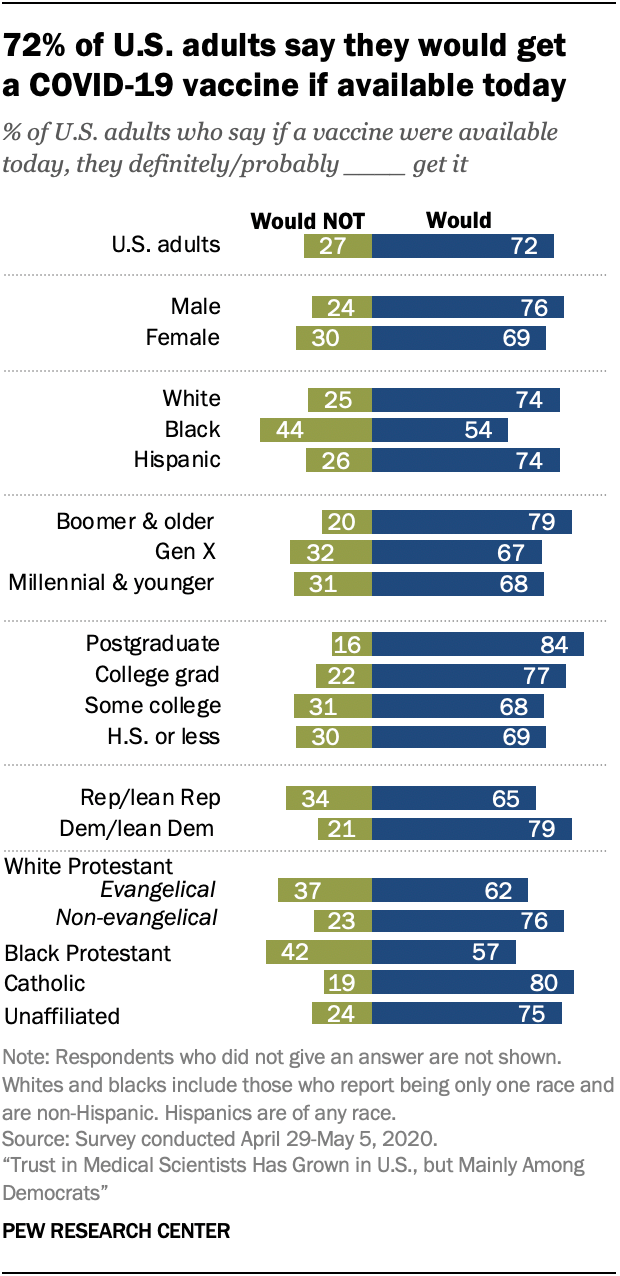 Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
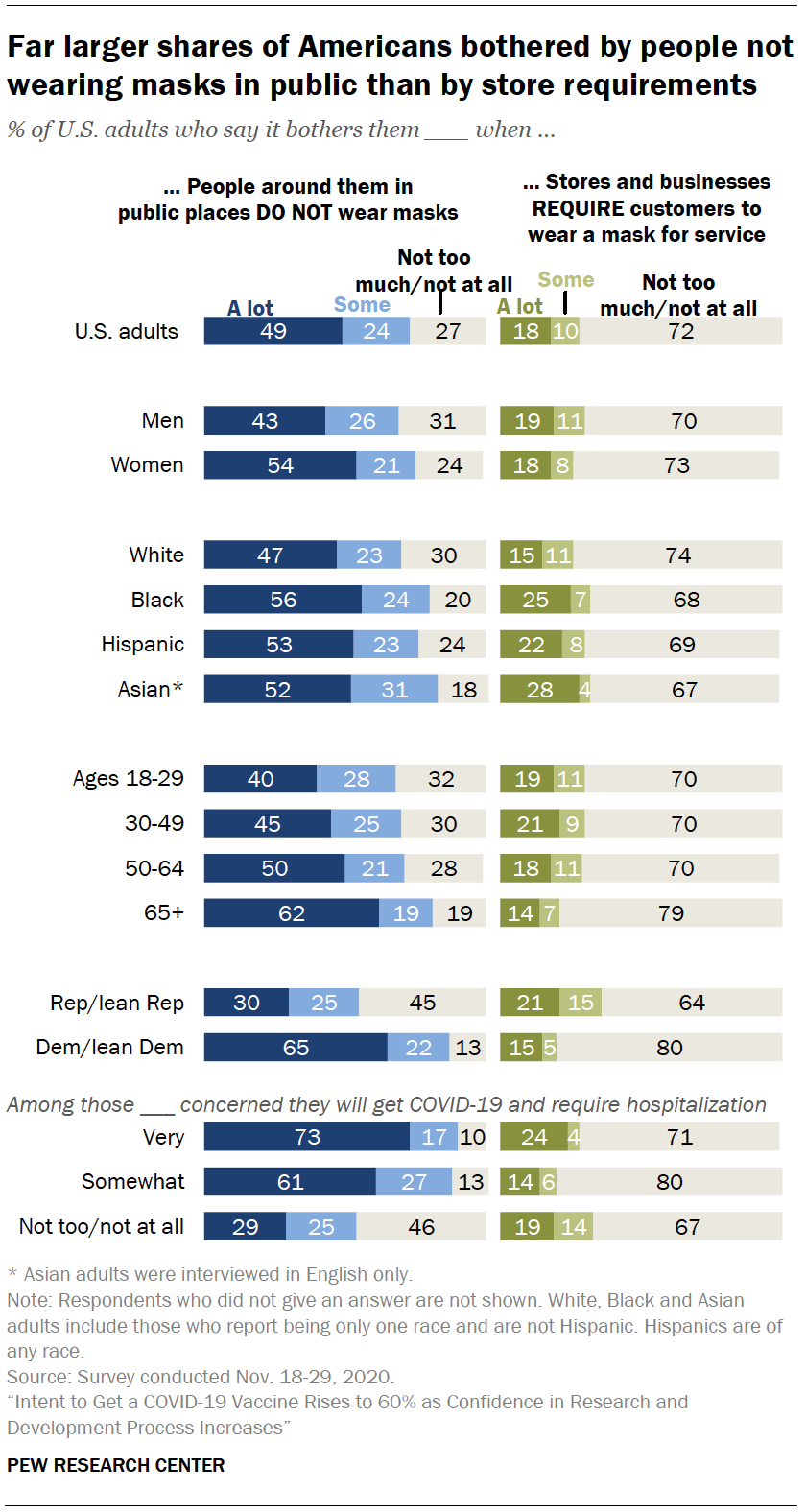 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
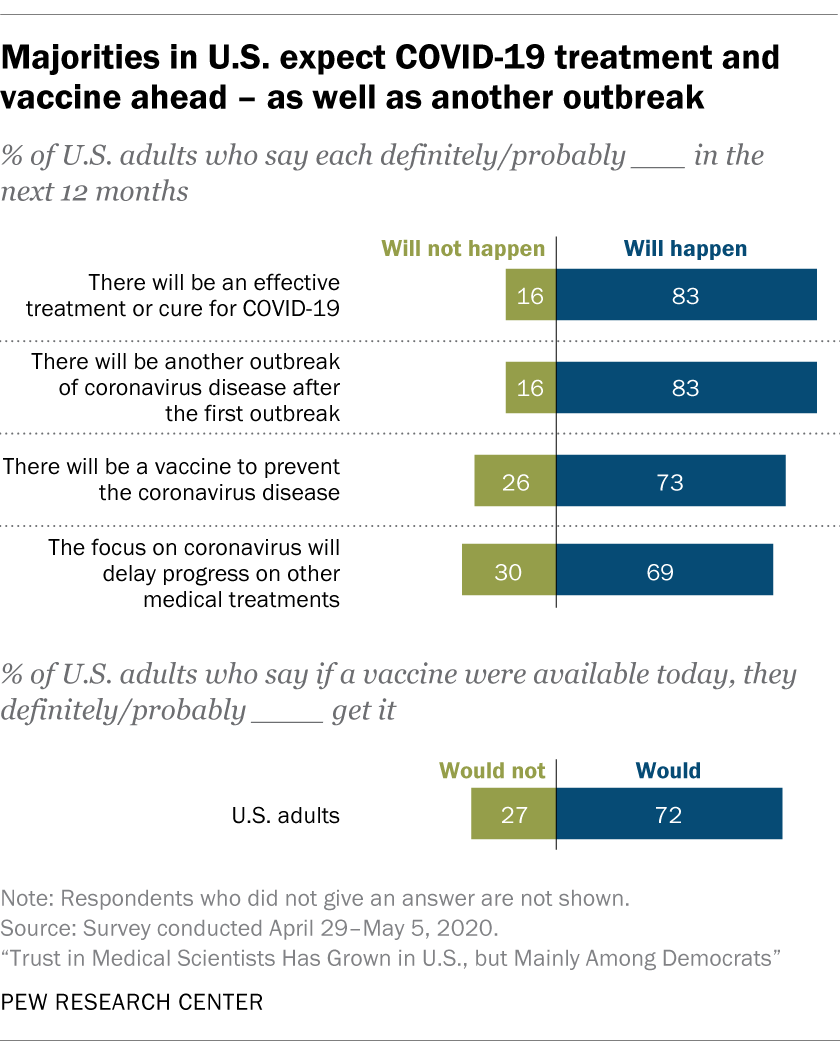 Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
Most Americans Expect A Covid 19 Vaccine Within A Year 72 Say They Would Get Vaccinated Pew Research Center
 The Most Promising Covid 19 Vaccine Candidates Covid 19 Vaccine Research
The Most Promising Covid 19 Vaccine Candidates Covid 19 Vaccine Research
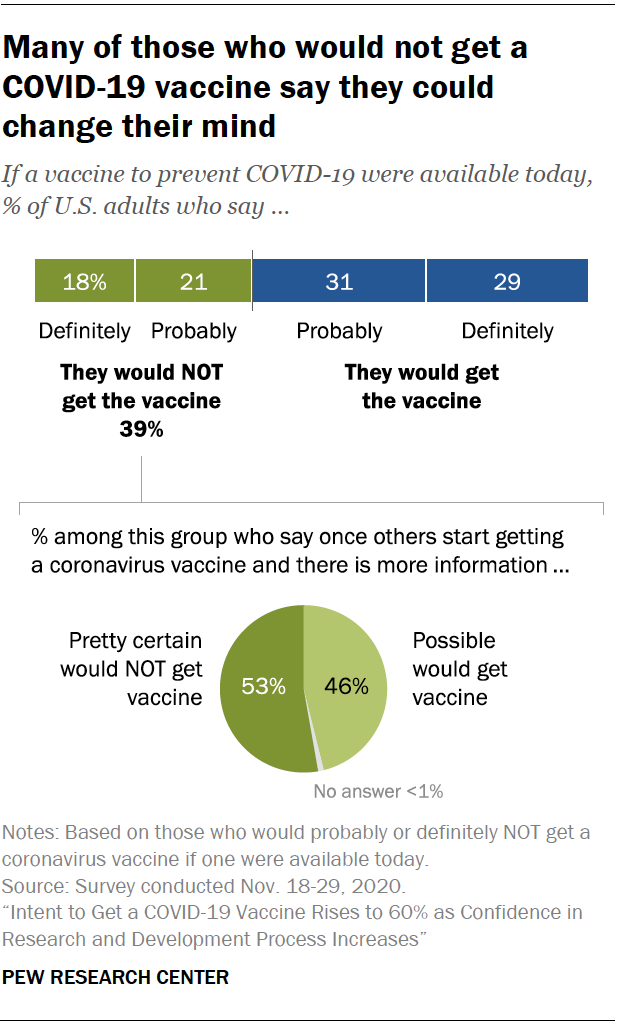 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
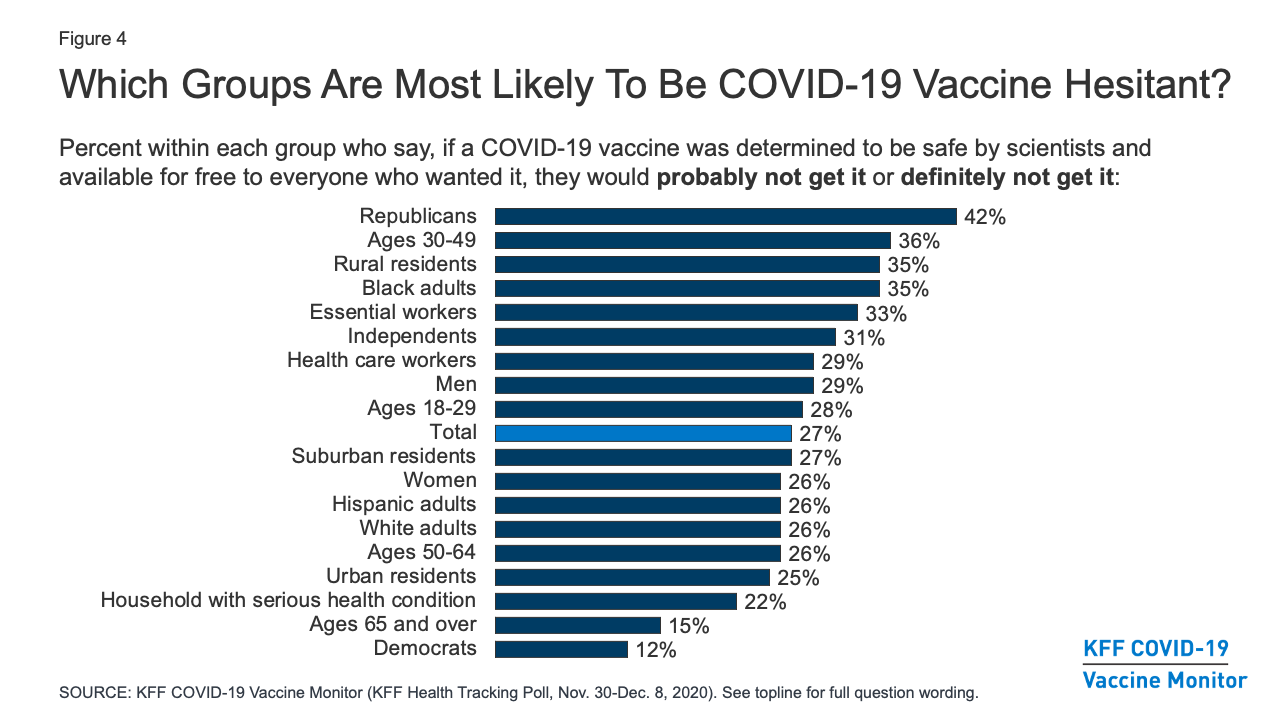 Kff Covid 19 Vaccine Monitor December 2020 Kff
Kff Covid 19 Vaccine Monitor December 2020 Kff
Post a Comment for "Will There Be A Covid 19 Vaccine In 2020"