Coronavirus Vaccine Trial Results Data
The new data included Covid-19 cases through April 9 and evaluated over 900 cases including more than 100 severe cases it said. The data collection and analysis are ongoing in order to allow up to two years of follow up on participants.
 Astrazeneca S Covid 19 Vaccine Holds Up In Updated Data Analysis Science News
Astrazeneca S Covid 19 Vaccine Holds Up In Updated Data Analysis Science News
In addition the analysis showed JJs vaccine candidate prevented hospitalizations or deaths.

Coronavirus vaccine trial results data. Data from 43448 participants half of whom received BNT162b2 and half of whom received placebo showed that the vaccine candidate was well tolerated and demonstrated 95 efficacy in. Covid-19 has killed more people than flu in a given season and is. Thursday December 10 2020 - 1021am.
3 Min Read MOSCOW Reuters - Preliminary results from the late-stage human trial of Russias main coronavirus vaccine candidate could include data from 5000-10000 participants Denis Logunov a. The vaccine is currently authorized for. For the most part these numbers match what the clinical trials showed.
According to the preliminary analysis JJs vaccine candidate was 66 effective at preventing both moderate and severe Covid-19 cases 28 days after trial participants received their vaccinations. 16 Moderna announced the first preliminary data from the trial followed by the complete data on Nov. Cases of COVID19 starting 14 days after Dose 2 were defined as symptomatic COVID19 requiring positive RT-PCR result and at least two systemic symptoms or one respiratory symptom.
Based on the data the CDC puts the chances of post-vaccination infection at 1 in 11000. The Janssen vaccine is a recombinant vector vaccine that uses a human adenovirus to express the SARS-CoV-2 spike protein. 2 days agoOcugens COVID-19 Vaccine Co-Development Partner Bharat Biotech Shares Second Interim Results demonstrating 100 Protection against Severe Disease including Hospitalization.
23 2020 is being conducted as part of the federal COVID-19 response. Earlier this month the director of the Chinese Center for Disease Control and Prevention said the country was formally considering giving people COVID-19 vaccine doses. The researchers estimated that the vaccine had an efficacy rate of 941 percent.
There were 11 COVID19 cases in the Moderna COVID19 Vaccine group and 185 cases in the placebo group with a vaccine efficacy of 941 95 confidence interval of 893 to 968. Chinese researchers are testing the mixing of COVID-19 vaccine doses developed by CanSino Biologics and a unit of Chongqing Zhifei Biological Products according to clinical trial registration data. The ENSEMBLE trial which began Sept.
2 days agoThe vaccine was 67 percent effective on average against moderate to severecritical COVID-19 at least 14 days after administration and 66 percent effective at 28 days after vaccination. 2 days agoThe Covid-19 vaccine trials have been published in peer reviewed journals. SARS-CoV-2 is the virus that causes COVID-19.
When looking only at severe cases of Covid-19 the vaccine was 85 the analysis found. Pfizer and BioNTech Announce Publication of Results from Landmark Phase 3 Trial of BNT162b2 COVID-19 Vaccine Candidate in The New England Journal of Medicine. Pfizer said its trial data showed the vaccine to be 95 effective against the novel coronavirus and Moderna said its vaccine was 94 effective in stopping.
Leading Coronavirus Treatments Include Antibodies Plasma Interferon
 Covid 19 Vaccine From Pfizer And Biontech Is Strongly Effective Data Show
Covid 19 Vaccine From Pfizer And Biontech Is Strongly Effective Data Show
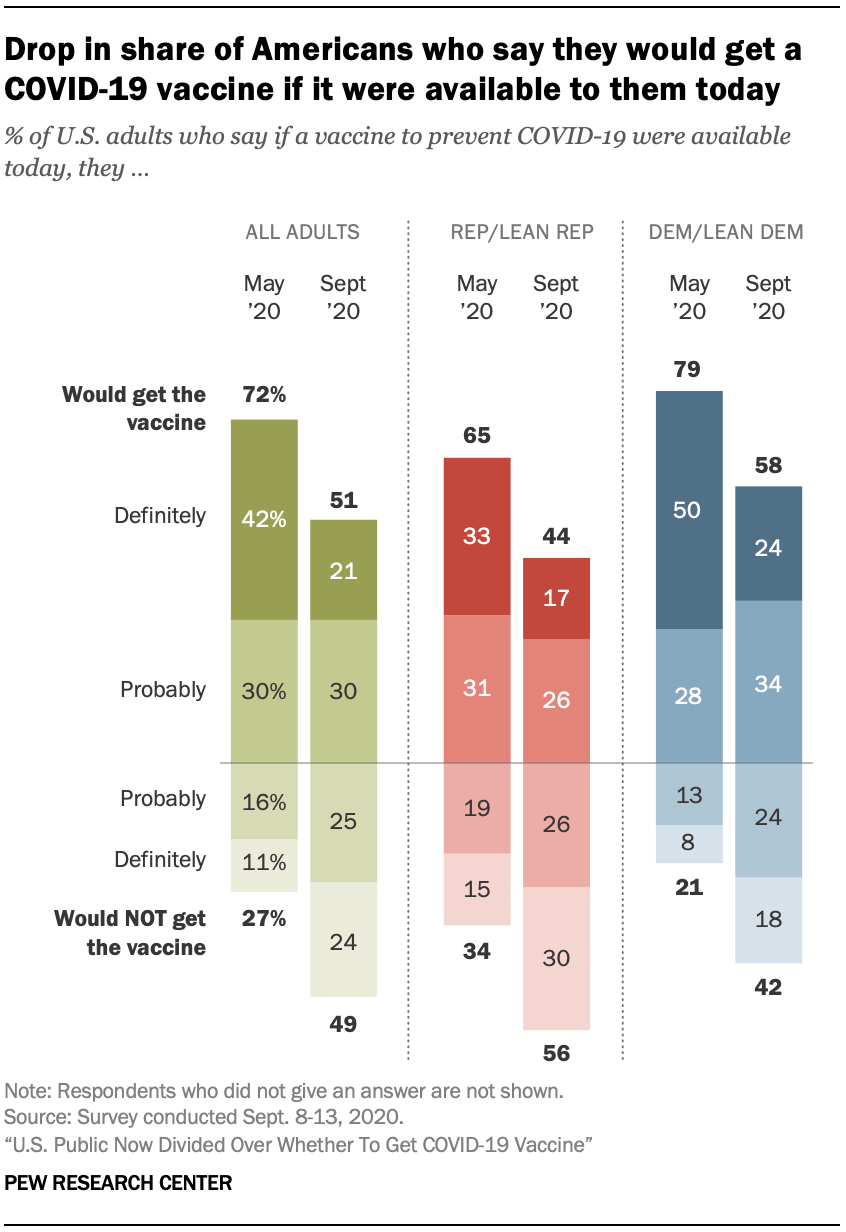 U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
U S Public Now Divided Over Whether To Get Covid 19 Vaccine Pew Research Center
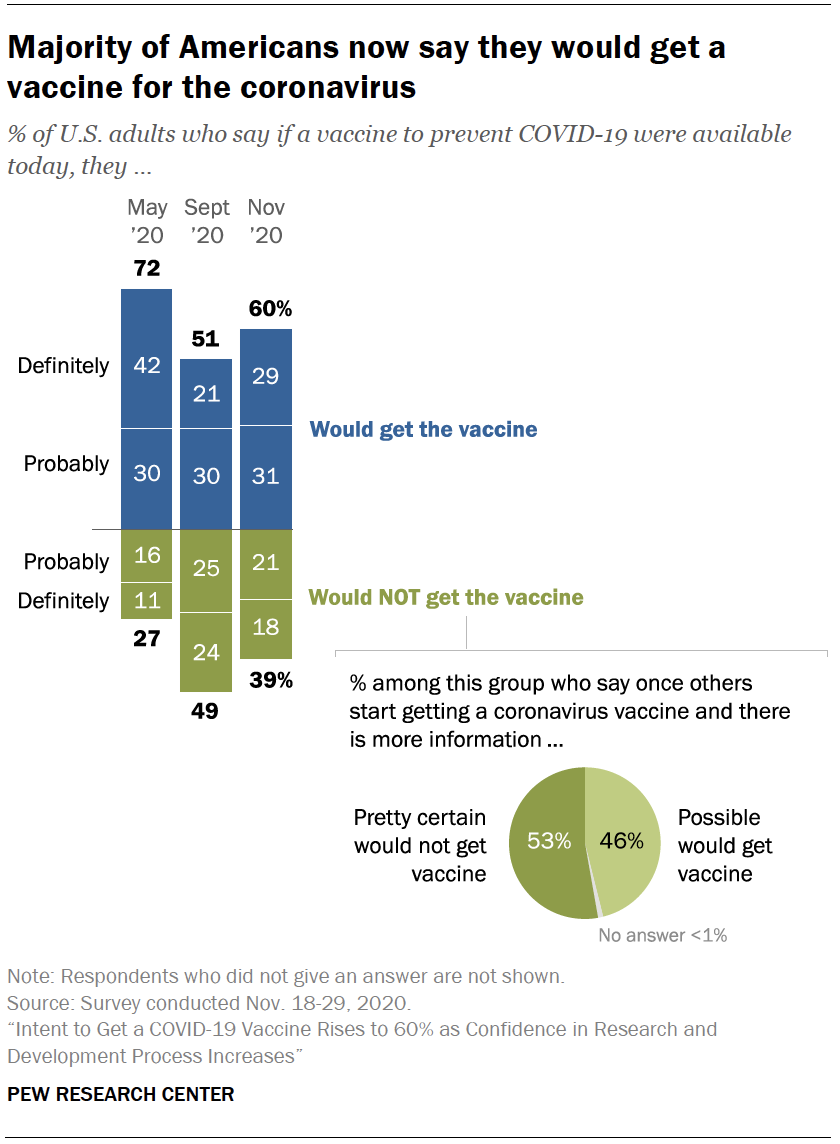
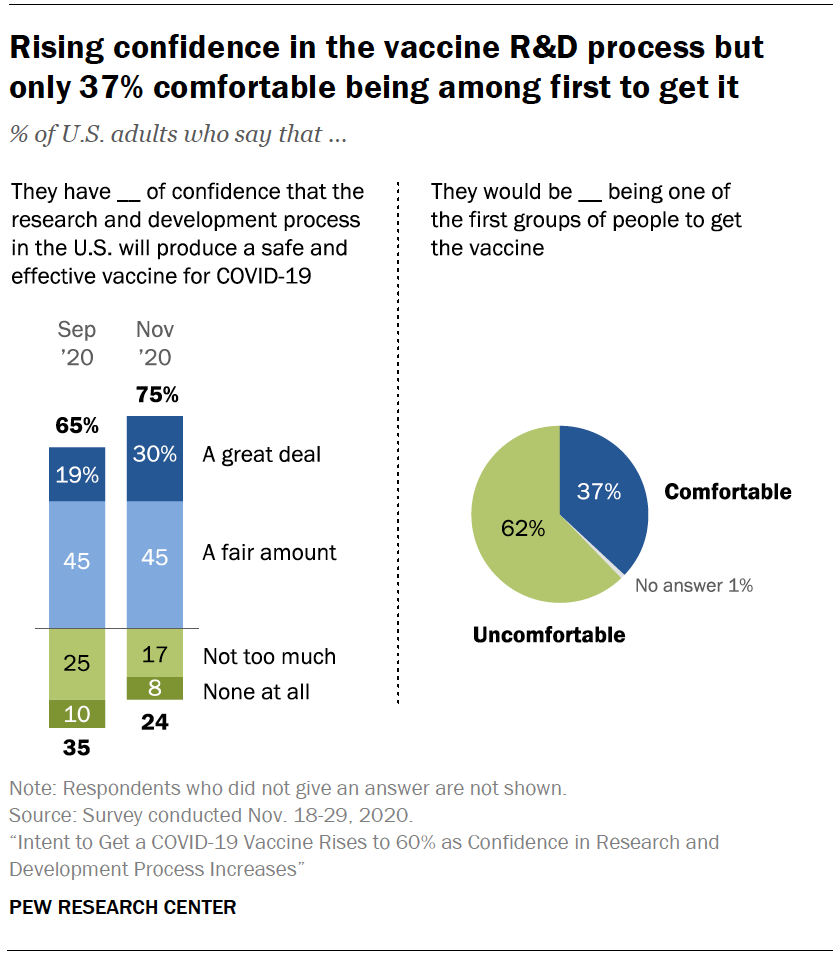 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
 First Data For Moderna Covid 19 Vaccine Show An Immune Response Stat
First Data For Moderna Covid 19 Vaccine Show An Immune Response Stat
 New Moderna Covid 19 Vaccine May Remain Effective For A Year Observer
New Moderna Covid 19 Vaccine May Remain Effective For A Year Observer
 Gilead Data Suggests Coronavirus Patients Are Responding To Treatment
Gilead Data Suggests Coronavirus Patients Are Responding To Treatment
 Initial Israeli Data First Pfizer Shot Curbs Infections By 50 After 14 Days The Times Of Israel
Initial Israeli Data First Pfizer Shot Curbs Infections By 50 After 14 Days The Times Of Israel
Chart Israeli Vaccination Data Cause For Optimism Statista
 Chart Israeli Vaccination Data Cause For Optimism Statista
Chart Israeli Vaccination Data Cause For Optimism Statista
 Pfizer Vaccine Results Are Promising But Lack Of Data Very Concerning Experts Say
Pfizer Vaccine Results Are Promising But Lack Of Data Very Concerning Experts Say
 Treatments And A Vaccine For Covid 19 The Need For Coordinating Policies On R D Manufacturing And Access
Treatments And A Vaccine For Covid 19 The Need For Coordinating Policies On R D Manufacturing And Access
 Hydroxychloroquine For Covid 19 What Do The Clinical Trials Tell Us The Centre For Evidence Based Medicine
Hydroxychloroquine For Covid 19 What Do The Clinical Trials Tell Us The Centre For Evidence Based Medicine
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Learn More About The Phase 3 Studies Of Our Investigational Covid 19 Vaccine Candidate And Our Commitment To Safety And Transparency Johnson Johnson
Learn More About The Phase 3 Studies Of Our Investigational Covid 19 Vaccine Candidate And Our Commitment To Safety And Transparency Johnson Johnson
 Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
Intent To Get A Covid 19 Vaccine Rises To 60 As Confidence In Research And Development Process Increases Pew Research Center
 Community Impact Data 3 New Covid Vaccines And Trials In Children A Month Of Dilemmas And Good News Absolutely Maybe
Community Impact Data 3 New Covid Vaccines And Trials In Children A Month Of Dilemmas And Good News Absolutely Maybe
 Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
 Fda Scientists Endorse Moderna Covid Vaccine Amid New Hints On Efficacy
Fda Scientists Endorse Moderna Covid Vaccine Amid New Hints On Efficacy
Post a Comment for "Coronavirus Vaccine Trial Results Data"