Covid Vaccine Breastfeeding Study Enrollment
Based upon available data it appears safe to get the COVID-19 vaccine if you are nursing a baby. Although the vaccines have not been studied in nursing mothers lactating women should be offered the COVID-19 vaccine.
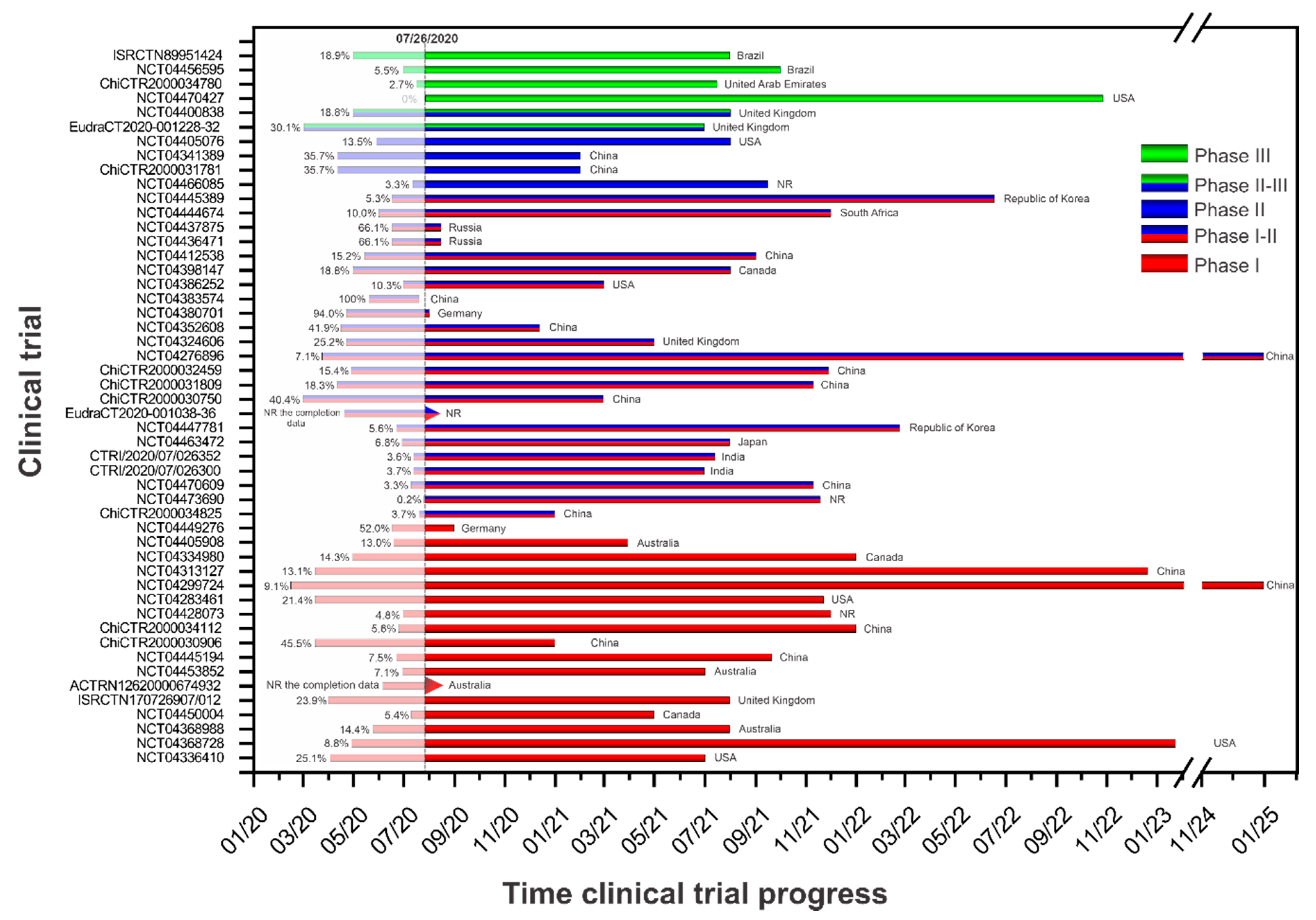 Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Food and Drug Administration FDA but has been authorized for emergency use by FDA under an Emergency Use Authorization EUA to prevent Coronavirus Disease 2019 COVID.

Covid vaccine breastfeeding study enrollment. Researchers are looking for 30000 participants to take part in the study. The Moderna and Johnson Johnson vaccines can only be given to individuals age 18 years and older Vaccination of individuals younger than 16 will start as. 1 day agoA study of more than 2000 pregnant women from dozens of hospitals around the world has found that those with COVID-19 saw a significantly higher risk of.
The trial is designed to evaluate the safety of mRNA-1273 and to determine if the vaccine can prevent symptomatic COVID-19 after two doses. 1 day agoThe study authors led by the CDCs Dr. Care for Breastfeeding People.
The vaccines do not contain live virus so being vaccinated does not pose a risk to the baby. RNA-Based COVID-19 Vaccines US IND Number. The Pfizer-BioNTech COVID-19 vaccine has not been approved or licensed by the US.
Despite this COVID-19 vaccines are not thought to be a risk to the breastfeeding infant and the benefits of breastfeeding are well known. But a new study offers some reassuring data for those who are pregnant or breastfeeding. Objectives To assess the reactogenicity measure the adaptive immune responses and track the long-term immune memory in 11-16-year-old young adolescents and their parents receiving the 3 COVID-19 vaccines-BNT162b2 CoronaVac and AZD1222-chosen by the Hong Kong Government.
Tom Shimabukuro said continued monitoring and more evidence is needed including on women who get COVID-19. To determine whether highly allergic people or people with mast cell disorders a buildup of the white blood cells that release substances causing symptoms similar to an allergic reaction are more likely to have an allergic reaction to the new Pfizer-BioNTech and Moderna COVID-19 vaccines. Because of this JCVI has recommended that the vaccine.
The COVID-19 pandemic continues to rapidly evolve posing unprecedented challenges for you and your patients. ACOG is here to support you by keeping you informed on the latest data so you can provide the best care helping you protect your practices through financial relief advocating for your. Participants were recruited from all of Israel between December 23 2020 and January 15 2021 through advertisements and social media.
Interim Guidance on Breastfeeding and Breast Milk Feeds in the Context of COVID-19 This interim guidance is intended for healthcare providers and lactation specialists who care for breastfeeding people and their infants and children who receive breast milk feeds during the COVID-19 pandemic. Participants must be at least 18 in good or stable health have an increased risk of. Individuals 16 or older who live work or study in Riverside County Teenagers 16 and 17 years old must be accompanied by a parent or legal guardian and register for a vaccination clinic that offers the Pfizer vaccine.
We conducted a prospective cohort study of a convenience sample of breastfeeding women either exclusive or partial belonging to vaccine-target groups who chose to be vaccinated. To compare the reactogenicity and immunogenicity across the 3 vaccines used for these children and. As secondary goals the trial also aims to study whether the vaccine can prevent severe COVID-19 or laboratory-confirmed SARS-CoV-2 infection with or without disease symptoms.
A Phase 123 Study to Evaluate the Safety Tolerability Immunogenicity and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals. Previous participation in a study to evaluate a COVID-19 vaccine or prior exposure to a COVID-19 vaccine. Pregnant and breastfeeding people were intentionally left out of clinical trials for the COVID-19 vaccines.
Use of immunosuppressant therapy regimens within the six months prior to enrollment in the study for planned use within the two years following enrollment.
Covid 19 Immune Response Markers In Breastmilk Umass Breastmilk Labumass Breastmilk Lab
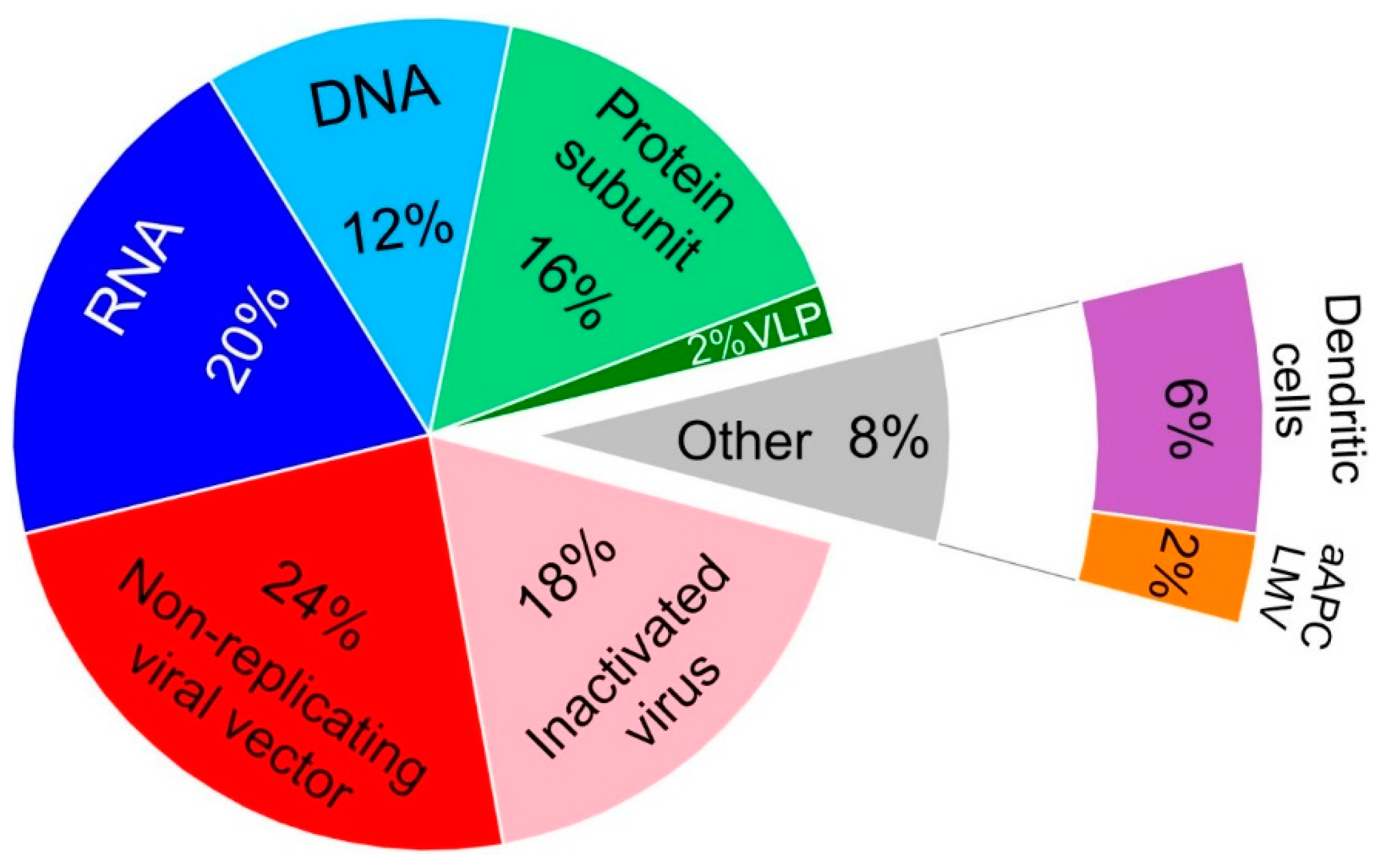 Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
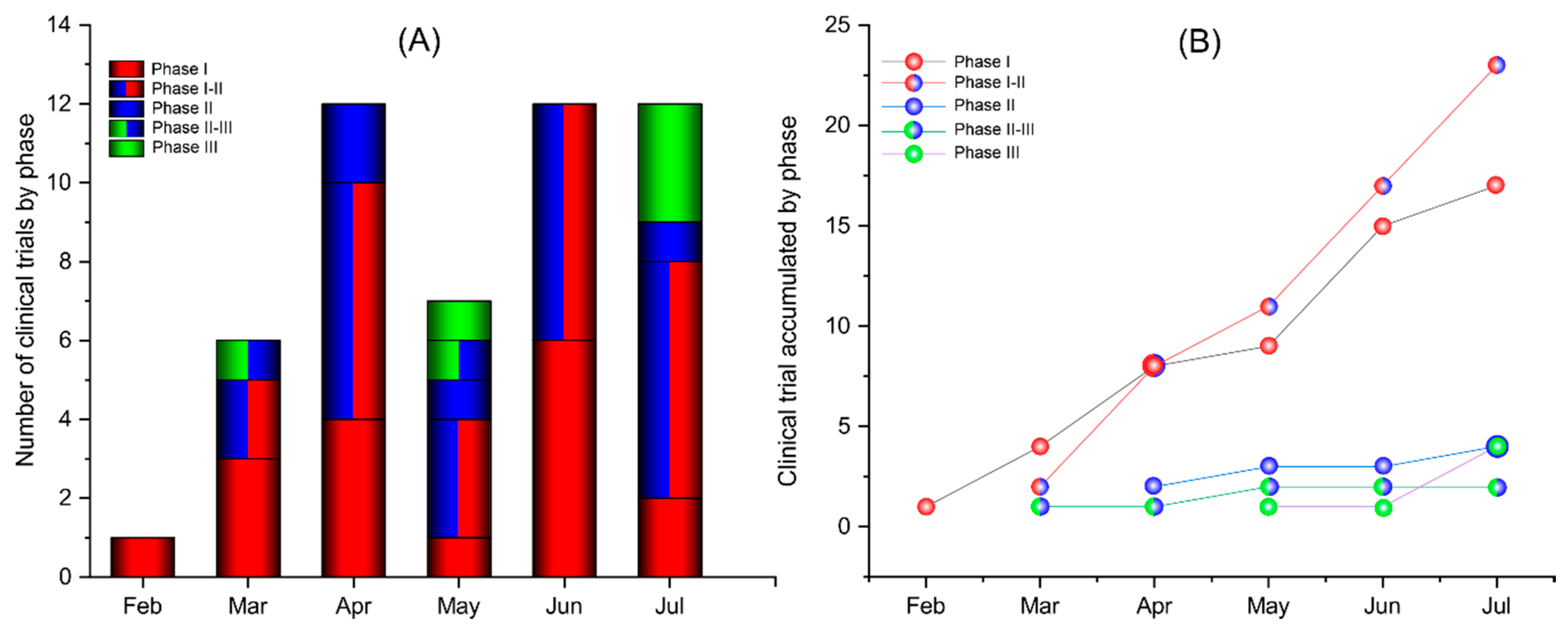 Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Covid 19 Information Updates Greenbelt Md
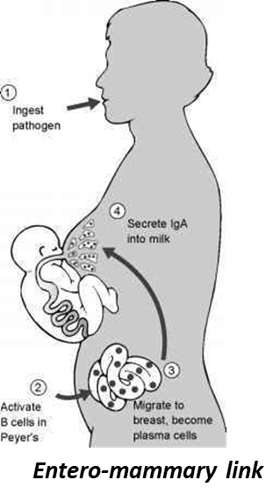 Research Rebecca Powell Laboratory
Research Rebecca Powell Laboratory
 Covid 19 Mrna Vaccines Orleans Community Health
Covid 19 Mrna Vaccines Orleans Community Health
 Vaccine Resources Gunnison County Coronavirus Resources
Vaccine Resources Gunnison County Coronavirus Resources
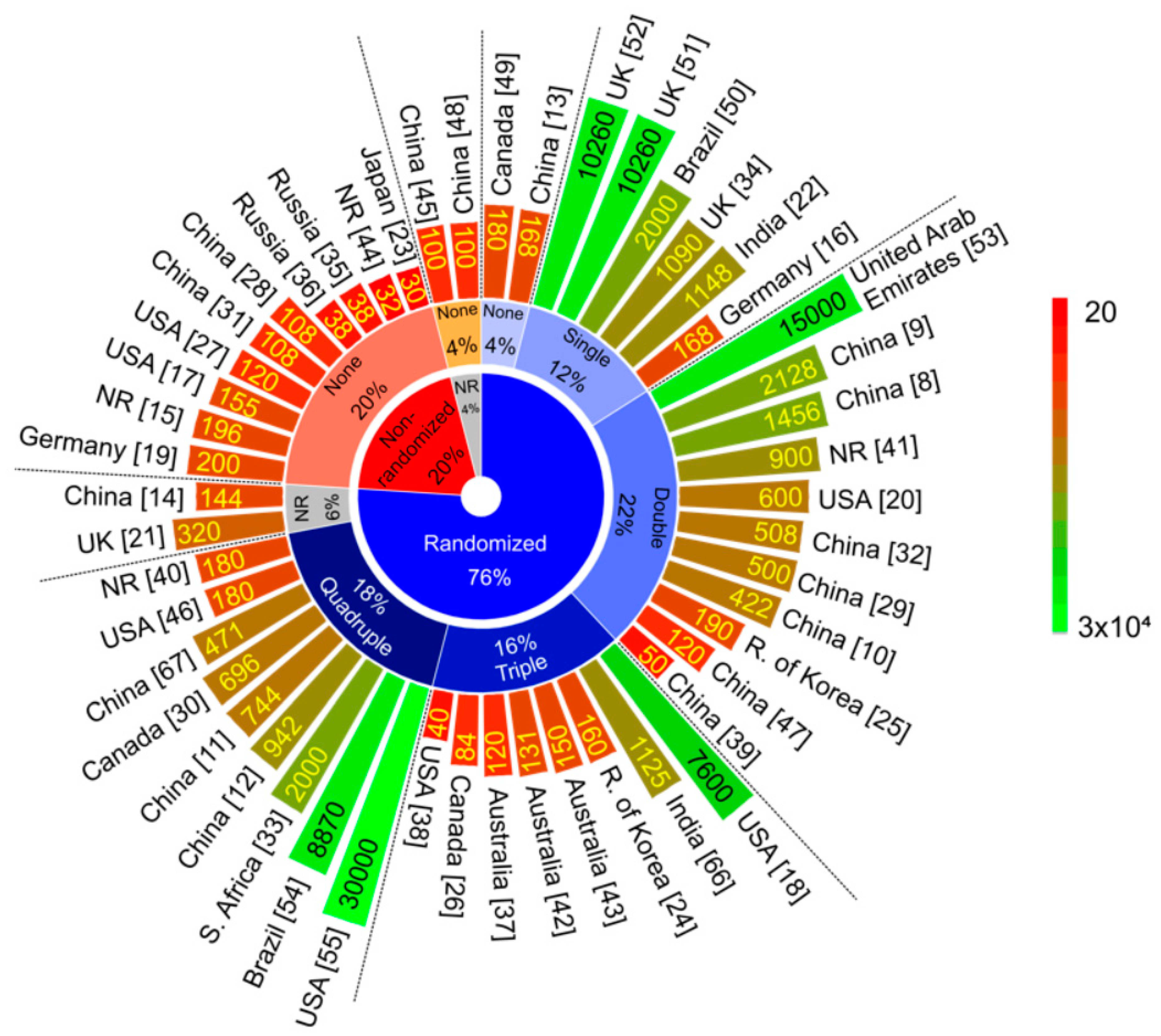 Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2 Html
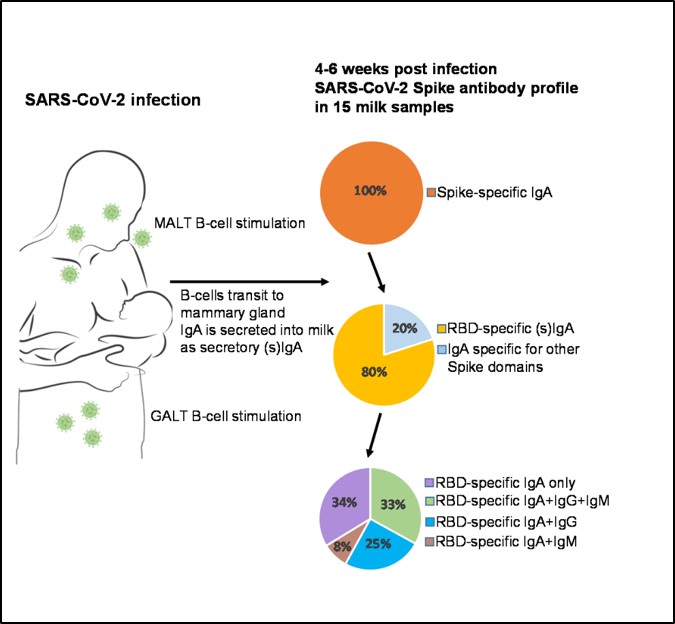 Research Rebecca Powell Laboratory
Research Rebecca Powell Laboratory
 Covid 19 Vaccine What You Need To Know Children S Hospital Of Richmond At Vcu
Covid 19 Vaccine What You Need To Know Children S Hospital Of Richmond At Vcu
 Can You Be In The Johnson Johnson Covid 19 Vaccine Trial If You Have An Autoimmune Condition
Can You Be In The Johnson Johnson Covid 19 Vaccine Trial If You Have An Autoimmune Condition
 Covid 19 Vaccine Information For The General Public Delaware S Coronavirus Official Website
Covid 19 Vaccine Information For The General Public Delaware S Coronavirus Official Website
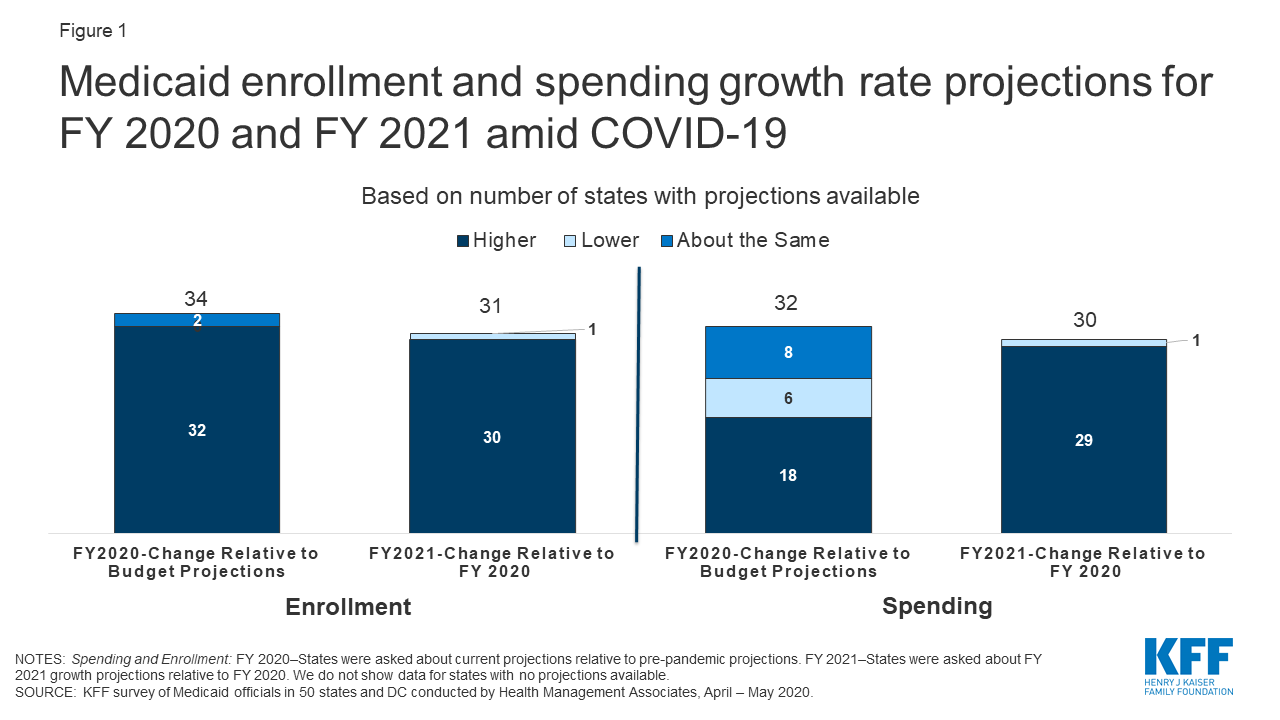 Early Look At Medicaid Spending And Enrollment Trends Amid Covid 19 Kff
Early Look At Medicaid Spending And Enrollment Trends Amid Covid 19 Kff
 Study Breast Milk Of Moms Vaccinated Against Covid 19 Contains Protective Antibodies For At Least 80 Days Coronavirus Stltoday Com
Study Breast Milk Of Moms Vaccinated Against Covid 19 Contains Protective Antibodies For At Least 80 Days Coronavirus Stltoday Com
 Breast Milk Of Vaccinated Mothers May Protect Babies From Covid 19 Study Suggests Wjbf
Breast Milk Of Vaccinated Mothers May Protect Babies From Covid 19 Study Suggests Wjbf
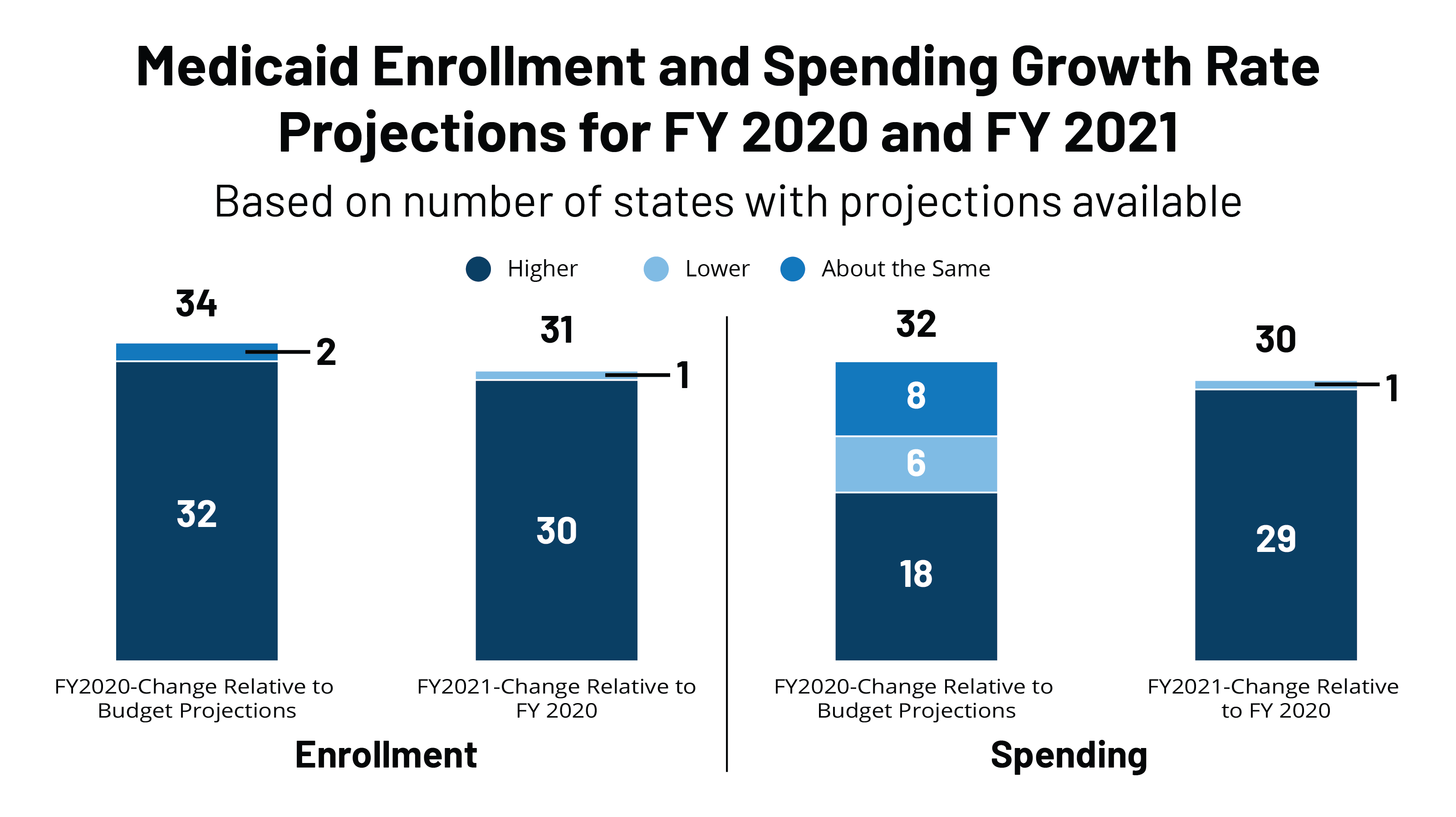 Early Look At Medicaid Spending And Enrollment Trends Amid Covid 19 Kff
Early Look At Medicaid Spending And Enrollment Trends Amid Covid 19 Kff



Post a Comment for "Covid Vaccine Breastfeeding Study Enrollment"