Moderna Covid Vaccine Phase 1 Results
Moderna Phase 1 results show coronavirus vaccine safe induces immune response. Moderna Incs experimental vaccine for COVID-19 showed it was safe and provoked immune responses in.
 Moderna S Covid 19 Vaccine Shows Positive Results Moves To Larger Studies
Moderna S Covid 19 Vaccine Shows Positive Results Moves To Larger Studies
Investigators observed 236 cases of symptomatic COVID-19 among participants at least 14 days after they received their first shot with 225 cases in the placebo group and 11 cases in the group receiving mRNA-1273.

Moderna covid vaccine phase 1 results. Moderna Phase 1 results show coronavirus vaccine safe induces immune response. Moderna Phase 1 results show coronavirus vaccine safe induces immune response Reuters Published July 15 2020 Moderna started its phase 2 trial in May and expects to start a. The Moderna vaccine was 941 effective at preventing symptomatic Covid-19 after the second dose.
Moderna Phase 1 results show COVID19 vaccine safe induces immune response Moderna shares jumped more than 15 in after-hours trading on Tuesday. CHICAGO Reuters - Moderna Incs MRNAO experimental vaccine for COVID-19 showed it. Moderna Announces Positive Interim Phase 1 Data for its mRNA Vaccine mRNA-1273 Against Novel Coronavirus.
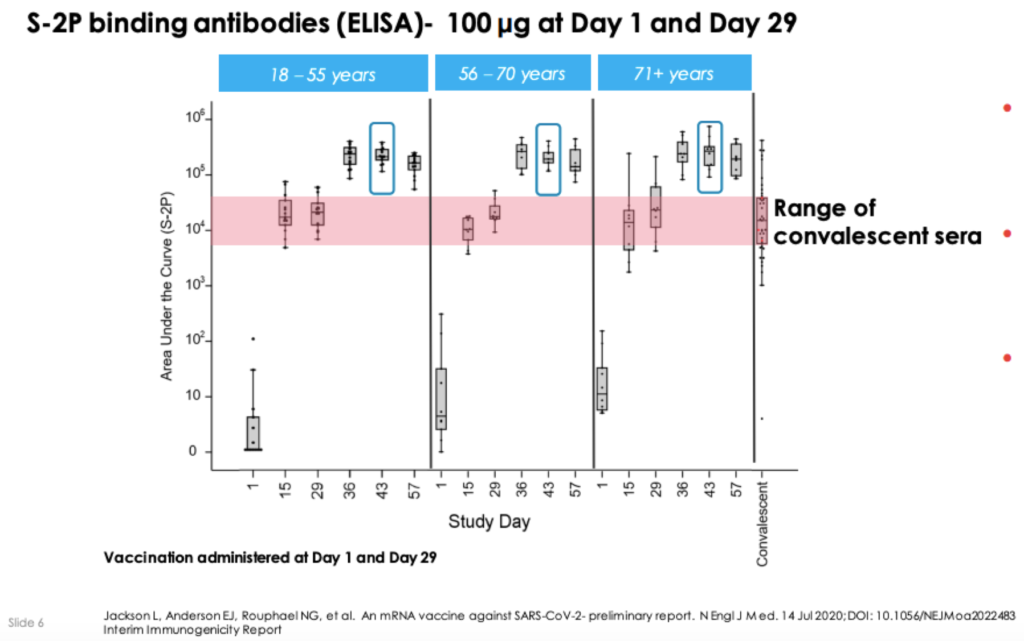
Modernas COVID-19 vaccine shows positive results moves to larger studies. The incidence of symptomatic COVID-19 was 941 lower in those participants who received mRNA-1273 as compared to those receiving placebo. After two doses all participants evaluated to date across the 25 µg and 100 µg dose cohorts seroconverted with binding antibody levels at or above levels seen in convalescent sera.
Moderna coronavirus vaccine shows promising safety and immune response results in published Phase 1 study but more research is needed. Illustrating the incredibly rapid pace of COVID-19 vaccine development biotechnology company Moderna has just revealed interim data from its Phase 1 human trials testing a novel mRNA vaccine. 21 hours agoIan Haydon was one of the first people to try out Modernas COVID-19 vaccine as part of the companys phase 1 trial with first and second doses administered in.
MRNA 159 after-hours as a study in the New England Journal of Medicine says the companys mRNA-1273 experimental vaccine for. Researchers reported on Tuesday. As of November 25 2020 among participants who had received at least 1 dose of vaccine or placebo vaccine15185 placebo15166 unsolicited adverse events that occurred within 28 days following each vaccination were reported by 239 of participants n3632 who received Moderna COVID19 Vaccine and 216 of participants n3277 who received placebo.
Moderna said Tuesday its Covid-19 vaccine was more than 90 effective at protecting against Covid and more than 95 effective against severe disease up. Early results from a phase 1 trial showed the vaccine was safe and prompted an immune response in humans. Moderna Phase 1 results show coronavirus vaccine safe induces immune response Moderna Incs experimental vaccine for COVID-19 showed it was safe and provoked immune responses in all 45 healthy.
MRNA-1273 elicited neutralizing antibody titer levels in all eight initial participants across the 25 µg. Moderna Incs experimental vaccine for COVID-19 showed it was safe and provoked immune responses in all 45 healthy volunteers in an ongoing early-stage study US. Jhonson Jhonson This virus is from the family of viruses that cause the common cold and is harmless to the human body.
Interim analysis of original cohorts of Phase 1 study evaluated two-dose vaccination schedule of mRNA-1273 across three dose levels 25 100 250 µg in 45 healthy adults. A preliminary report published yesterday in the New England Journal of Medicine on an ongoing phase 1 clinical trial of the mRNA-1273 COVID-19 vaccine developed by Moderna and study sponsor the US National Institute of Allergy and Infectious Diseases NIAID showed that it generated an immune response in healthy adults and was generally well tolerated. Moderna Announces Publication in The New England Journal of Medicine of Interim Results From Phase 1 Study of Its mRNA Vaccine Against COVID-19 mRNA-1273 July 14 2020 at 514 PM EDT.
 Summary Of Current Sars Cov 2 Vaccine Trials
Summary Of Current Sars Cov 2 Vaccine Trials
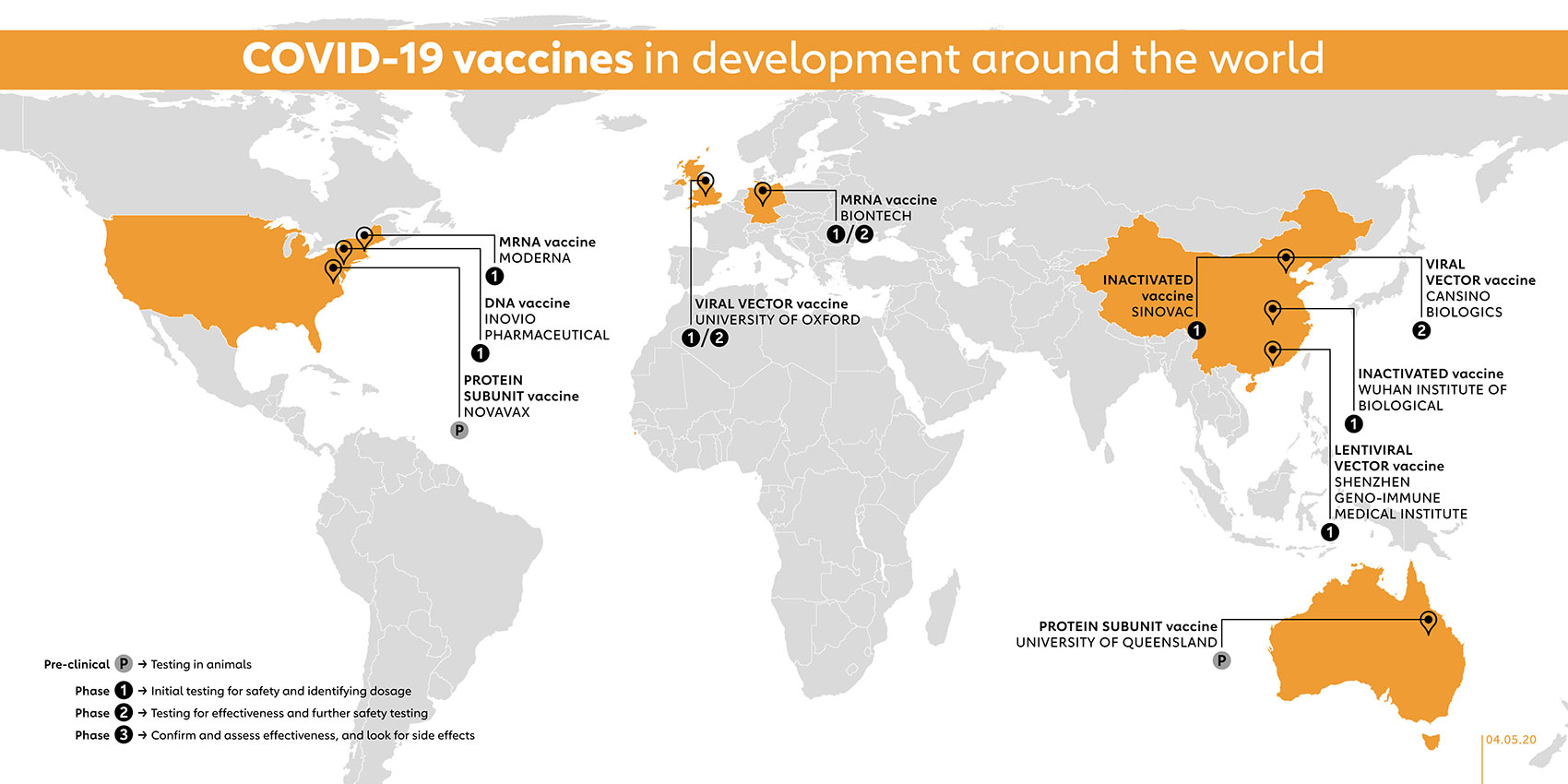 1 2 Update On Treatments And Vaccines Under Development
1 2 Update On Treatments And Vaccines Under Development
 Moderna S Coronavirus Vaccine Nearly 95 Effective Analysis Finds Shots Health News Npr
Moderna S Coronavirus Vaccine Nearly 95 Effective Analysis Finds Shots Health News Npr
 Moderna S Coronavirus Vaccine Nearly 95 Effective Analysis Finds Shots Health News Npr
Moderna S Coronavirus Vaccine Nearly 95 Effective Analysis Finds Shots Health News Npr
 Clinical Trial Shows Moderna Covid 19 Vaccine On Right Track The San Diego Union Tribune
Clinical Trial Shows Moderna Covid 19 Vaccine On Right Track The San Diego Union Tribune
 Moderna Covid 19 Vaccine Generates Immune Response Early Data Show
Moderna Covid 19 Vaccine Generates Immune Response Early Data Show
 Covid 19 Clinical Trials Recently Completed Showing Mixed Results
Covid 19 Clinical Trials Recently Completed Showing Mixed Results
 Here S How We Ll Know When A Covid 19 Vaccine Is Ready
Here S How We Ll Know When A Covid 19 Vaccine Is Ready
 Unwrapping The Biological Secrets Behind Moderna S Covid 19 Vaccine Technology Health Policy Watch
Unwrapping The Biological Secrets Behind Moderna S Covid 19 Vaccine Technology Health Policy Watch
 Moderna Announces Publication In The New England Journal Of Medicine Of Interim Results From Phase 1 Study Of Its Mrna Vaccine Against Covid 19 Mrna 1273 Azbio
Moderna Announces Publication In The New England Journal Of Medicine Of Interim Results From Phase 1 Study Of Its Mrna Vaccine Against Covid 19 Mrna 1273 Azbio
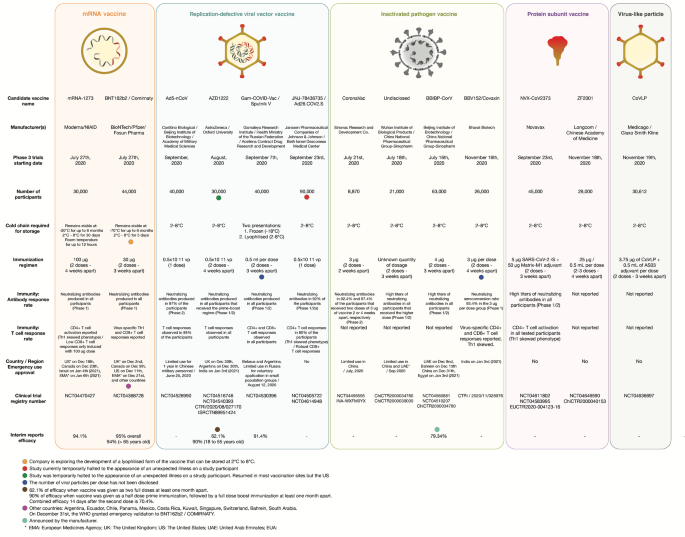 Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Http Dhss Alaska Gov Dph Epi Id Siteassets Pages Humancov Literature Covidvaccine Pdf
 The Clinical Trial Results Stampede Begins Covid 19 Vaccine Race Month 7 Absolutely Maybe
The Clinical Trial Results Stampede Begins Covid 19 Vaccine Race Month 7 Absolutely Maybe
 Covid 19 Vaccine Faq Anne Arundel County Department Of Health
Covid 19 Vaccine Faq Anne Arundel County Department Of Health
 Will Moderna Beat Oxford To Produce The First Covid 19 Vaccine
Will Moderna Beat Oxford To Produce The First Covid 19 Vaccine

Https Www Alabamapublichealth Gov Covid19 Assets Cov Pfizer Moderna Vaccine Trial Summary Pdf
 First Data For Moderna Covid 19 Vaccine Show An Immune Response Stat
First Data For Moderna Covid 19 Vaccine Show An Immune Response Stat

Post a Comment for "Moderna Covid Vaccine Phase 1 Results"