Is Quest Diagnostics Covid Antibody Test Fda Approved
In a response to KCRA 3 Quest Diagnostics said one of its two antibody tests are FDA EUA approved and that both tests range from 985 to 994 accuracy. Quest diagnostics coronavirus antibody test is first available for purchase online Walgreens stores started offering antibody testing from LabCorp in.
 Ortho Clinical Diagnostics And Quest Diagnostics To Broaden Availability Of Covid 19 Antibody Testing
Ortho Clinical Diagnostics And Quest Diagnostics To Broaden Availability Of Covid 19 Antibody Testing
Medical providers and testing capacity for the unfolding COVID-19 pandemic continues to be sorely tested.
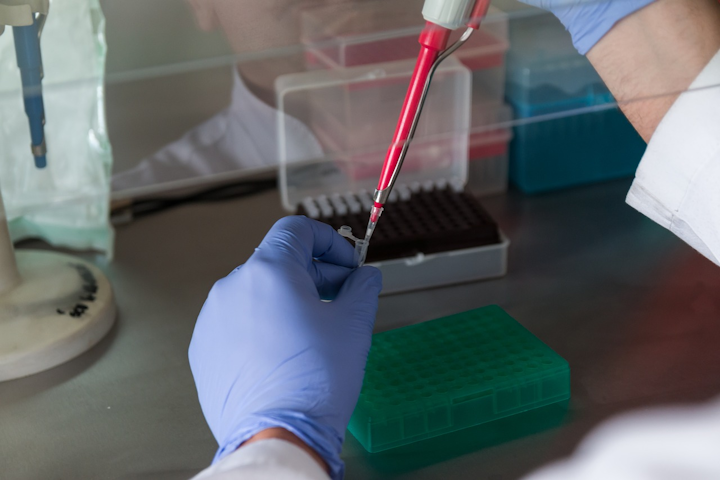
Is quest diagnostics covid antibody test fda approved. However dozens of other tests are being marketed in the US. A COVID-19 antibody test also known as a serology test is a blood test that can detect if a person has antibodies to SARS-CoV-2 the virus that causes COVID-19. This means that while Quest Diagnostics has validated the test and has the data to.
The FDA has approved Quests blood tests for emergency use during the coronavirus pandemic. Quest Diagnostics is rolling out its own antibody testing service using tests from. As the FDA has indicated antibody testing has the potential to help healthcare professionals identify people who have been exposed to COVID-19 and who have developed an immune response said.
Quests test has been approved by the FDA. All Massachusetts-based American Family. Heres a look at which local care centers are offering testing.
Food and Drug Administration has allowed companies to sell antibody tests without federal authorization. The serology antibody tests run by Quest Diagnostics are either approved by the FDA under an emergency use authorization or were released for use under the FDA guidance Policy for Diagnostic. Companies must validate the tests accuracy and apply labels stating the.
But there is now a new route for people to pursue serological testing to detect who has already been exposed to the SARS-CoV-2 virus. Major lab testing provider Quest Diagnostics Inc. COVID-19 antibody tests can help.
1 Testing for SARS-CoV-2 can play an important role in the fight against COVID-19 including for individuals who may be asymptomatic or who are 10 days postSARS-CoV-2 exposure or postsymptom onset. The Associated Press contributed to. FDA has authorized the intended use of these antibody tests to indicate whether a patient has had recent or prior COVID-19 infection.
On April 28 2020 FDA issued an umbrella EUA for SARS-CoV-2 Antibody Tests Lateral flow or Enzyme-linked immunosorbent assay ELISA tests that. 23 SARS-CoV-2 antibody testing can be used to. In the meantime several companies are working to expand the reach of antibody testing.
Gorode said the antibody tests used by Quest meet FDA standards. Has launched a service to enable consumers to request tests via their own. The serology antibody tests run by Quest Diagnostics are either approved by the FDA under an emergency use authorization or were released for.
The serology antibody tests run by Quest Diagnostics are either approved by the FDA under an emergency use authorization or were released for use under the FDA. This test has been authorized by the Food and Drug Administration under an Emergency Use Authorization. FDA approves intravenous coronavirus antibody test Thomas Schinecker Roches head of diagnostics said the company aims to more than double production of tests.
Neither companys test has yet received an official authorization from the FDA but the agency is allowing antibody test developers to proceed without review with a few caveats. The Food and Drug Administration gave emergency approval to a COVID-19 antibody test that boasts near-perfect accuracy the company said. Under the revised rules for antibody tests manufacturers will have to provide validation data within 10.
The capacity of US.
 Testing For Covid 19 What You Need To Know June 8 Update
Testing For Covid 19 What You Need To Know June 8 Update
 Quest Diagnostics Moves Toward Antibody Testing For Covid 19 Mddionline Com
Quest Diagnostics Moves Toward Antibody Testing For Covid 19 Mddionline Com
 Roche Gets Emergency Fda Approval For Fastest Covid 19 Diagnostic Test Yet Geneonline News
Roche Gets Emergency Fda Approval For Fastest Covid 19 Diagnostic Test Yet Geneonline News
 Covid 19 Testing For Employees Employers Quest Diagnostics
Covid 19 Testing For Employees Employers Quest Diagnostics
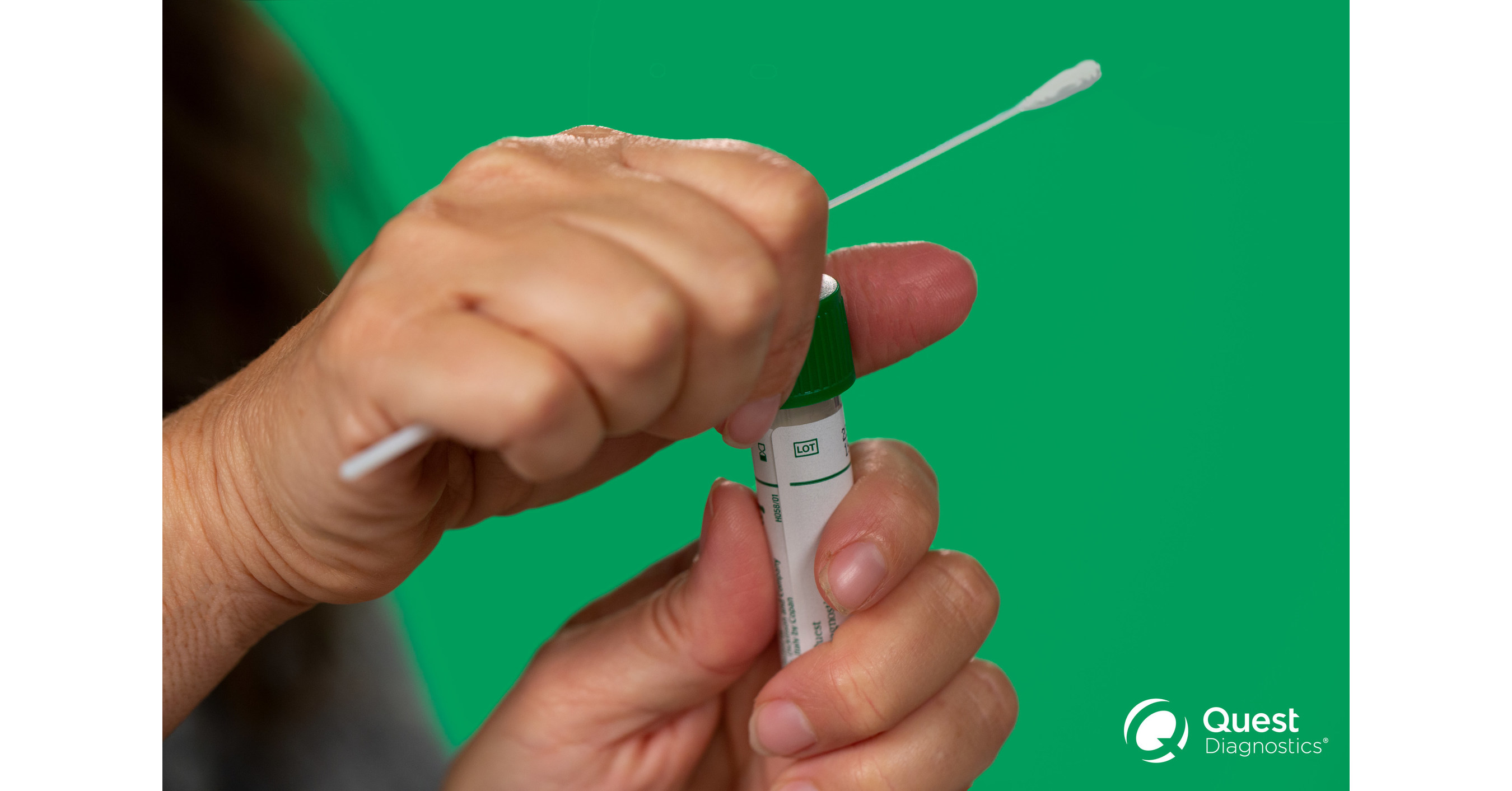 Fda Authorizes Quest Diagnostics Covid 19 Nasal Specimen Self Collection Kit For Emergency Use
Fda Authorizes Quest Diagnostics Covid 19 Nasal Specimen Self Collection Kit For Emergency Use
Https Www Fda Gov Media 136228 Download
 Covid 19 Information For Patients Sonora Quest
Covid 19 Information For Patients Sonora Quest
 Fda Authorizes Quest Diagnostics Covid 19 Nasal Specimen Self Collection Kit For Emergency Use
Fda Authorizes Quest Diagnostics Covid 19 Nasal Specimen Self Collection Kit For Emergency Use
Https Www Questdiagnostics Com Home Covid 19 Hcp Molecular And Serology Testing Information
How To Get Coronavirus Antibody Blood Test Online From Quest
 Quest Diagnostics Self Collection Covid 19 Test Kit Gets Fda Nod For Emergency Use Fiercehealthcare
Quest Diagnostics Self Collection Covid 19 Test Kit Gets Fda Nod For Emergency Use Fiercehealthcare
 Quest Diagnostics Launches Consumer Initiated Covid 19 Antibody Test Through Questdirect
Quest Diagnostics Launches Consumer Initiated Covid 19 Antibody Test Through Questdirect
Https Www Questdiagnostics Com Dms Documents Covid 19 Questcovid Antibodytestfacts Questcovid Antibodytestfacts Pdf
 Quest Diagnostics Launches Consumer Initiated Covid 19 Antibody Test Through Questdirect
Quest Diagnostics Launches Consumer Initiated Covid 19 Antibody Test Through Questdirect
 Covid 19 Information For Patients Sonora Quest
Covid 19 Information For Patients Sonora Quest
 Ortho Clinical Diagnostics And Quest Diagnostics To Broaden Availability Of Covid 19 Antibody Testing Medical Laboratory Observer
Ortho Clinical Diagnostics And Quest Diagnostics To Broaden Availability Of Covid 19 Antibody Testing Medical Laboratory Observer
 Roche Snags Fda Nod For Covid 19 Antibody Test 2020 05 04 Bioworld
Roche Snags Fda Nod For Covid 19 Antibody Test 2020 05 04 Bioworld


Post a Comment for "Is Quest Diagnostics Covid Antibody Test Fda Approved"